Herpes simplex virus
According to the World Health Organization (WHO), herpes simplex virus (HSV) is a prevalent virus, affecting nearly two-thirds of the world's population. Once inside the body, HSV forms a latent infection that periodically awakens, causing painful herpes simplex, usually around the nose and mouth or reproductive area. While HSV is just an annoyance to most people, for some it can also cause dangerous eye infections and brain inflammation, as well as life-threatening infections in newborns.
1. HSV Classification, Structure, and Genome
HSV is divided into two subtypes: HSV-1 and HSV-2. They share about 50% sequence homology. HSV-1 commonly causes oral herpes, resulting in cold sores or blisters around the mouth and ocular areas but sometimes progressing to more severe conditions including blindness, hearing loss, and encephalitis. HSV-2 is often responsible for genital herpes, causing itching, irritation, clear fluid blisters, and pain in the genital and anal areas. Both two HSV types can interchangeably infect oral or genital sites.
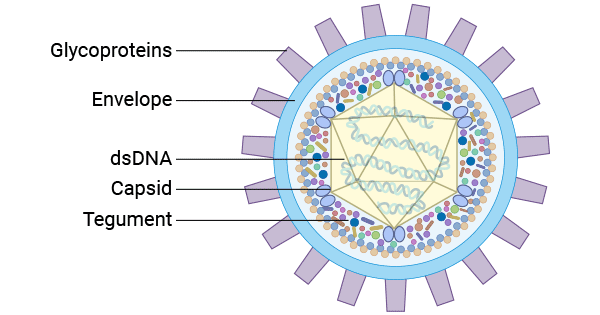
The mature infectious HSV is structurally composed of four prominent components from the core outward: a central large double-stranded linear DNA encapsulated within an icosahedral capsid enclosed by a layer of tegument comprising 22 viral proteins (VPs). Outermost, there is an outer envelope containing different glycoproteins, including glycoprotein B (gB), gC, gD, gE, gG, gH, gI, gJ, gK, gL, gM, and gN, in different shapes and sizes.
Figure 1. The HSV virion structure
This picture is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8923070/
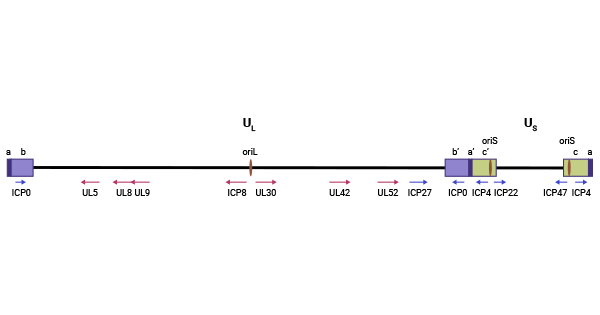
The HSV genome, spanning approximately 152 kbp, consists of long and short regions of unique regions (UL and US) flanked by inverted repeat sequences arrayed as ab-UL-b′a′c′-US-ca. These regions contain three replication origins: OriL and two copies of OriS. OriL is situated within the UL region, flanked by the ICP8 and UL30 genes, while the two copies of OriS are located in the inverted repeat short/terminal repeat short (IRS/TRS) region of the viral genome, positioned between the genes for the immediate early proteins ICP4 and ICP22/47, respectively.
Figure 2. Diagram of the HSV genome
This picture is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8539067/
2. HSV Cell Cycle
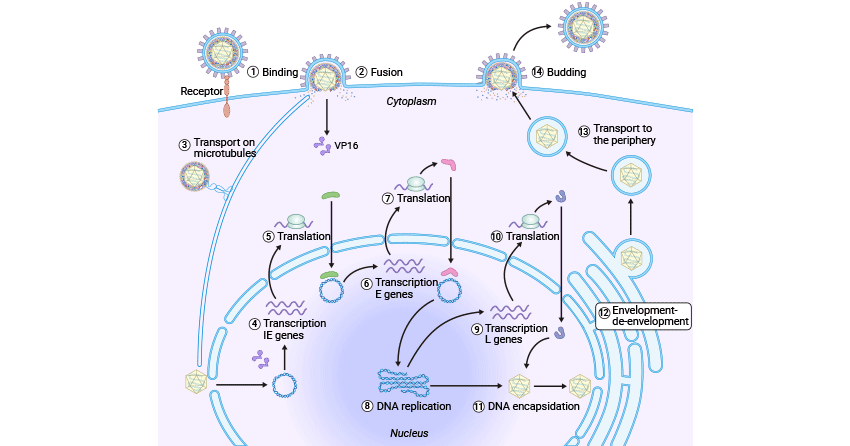
Like other viruses, the HSV cell cycle also includes entry, capsid Uncoating and viral DNA Release into the cell nucleus, biosynthesis, assembly, maturation, and release.
Figure 3. HSV cell cycle
This picture is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8539067/
2.1 HSV Entry into the Host Cell through Attachment and Fusion
The HSV recognizes and binds to the host cell surface receptor, heparan sulfate proteoglycans (HSPGs), through its viral protein gB and gC, allowing itself to attach to the host cell membrane. The HSV surfs along filopodia toward the cell body. The HSV gD binds to the host cell membrane receptors, including nectin1/2, HVEM, and 3-OH-HS, and undergoes conformational changes, promoting gB interacting with gH/gL heterodimer, thus inducing the viral envelop fusion with the host cell membrane [1,2].
Some studies also suggest that gM, gK, UL11, UL20, and so on are also involved in the fusion between viral envelop and host cell membrane.
| HSV Receptors |
| gD receptors |
HVEM (herpesvirus entry mediator) |
| Nectin-1 |
| Nectin-2 |
| 3-O-Sulfated Heparan Sulfate Proteoglycan (3-OS HS) |
| gB Receptors |
PILRα (paired immunoglobulin-like receptor α) |
| MAG (myelin-associated glycoprotein) |
| NMHC-IIA (non-muscle myosin heavy chain IIA) |
| gH/gL Receptors |
αvβ6 |
| αvβ8 |
2.2 Capsid Uncoating and Viral DNA Release into the Cell Nucleus
Upon fusion, the viral DNA-containing capsid and tegument are released into the cytoplasm of the host cell. The nucleocapsid is then transported to the cell nucleus along the microtubules through the interaction between UL36 and motor proteins [3,4], during which many tegument viral proteins dissociate from the capsid like VP16, while VP1/2 (UL36) and UL37 persist in association [5]. The resulting partially tegumented capsid is docked into the host nuclear pore (NPC), where the viral DNA is released into the host cell's nucleus.
2.3 Viral Gene Expression
After HSV's DNA entry into the nucleus, it is rapidly cycled by the host ligase. The UL48-encoding tegument protein VP16 recruits the host cell factor 1 (HCF1) and octamer binding protein 1 (Oct-1) to the viral immediately early (IE) gene promoter region, initiating the transcription of the five IE genes (α genes) including IPC0, ICP4, ICP22, ICP27, and Us1.5, which destroys the repressor complex and drives the transcription of early (E) genes (β genes). Among the E gene encoding products, seven proteins are involved in viral DNA replication. Once viral DNA replication occurs, late (L) genes (γ genes) are expressed, with a significant portion of these genes encoding structural proteins essential for virus assembly like procapsid.
Vhs (virion host shutoff protein) is an endoribonuclease encoded by the UL41 gene. It triggers rapid shutoff of host cell protein synthesis, disrupts preexisting polysomes, and accelerates the degradation of host mRNAs in the absence of de novo viral gene expression.
2.4 Genomic Replication
The genome replicates in a bidirectional or through θ manner, producing many circular progeny DNA during the viral early replication. At the later stage of DNA replication, DNA synthesis turns to rolling circle replication (σ replication), forming a large number of branched, head-to-tail concatemers of the viral genome, which are known as immature DNA and are the best substrate for assembly.
HSV DNA synthesis requires at least seven proteins, including UL9 (origin-binding protein), ICP8 (single-strand DNA-binding protein, UL29), UL30 (polymerase), UL42 processivity factor, and UL5/UL8/UL52 (helicase-primase complex), the later six of which play core replication roles at the replication fork. Several proteins acting as auxiliary factors not essential for HSV DNA replication, including UL23, UL39, UL40, UL2, UL50, and UL12.
This Table information is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3428768/
2.5 DNA Packaging and Release
The resultant head-to-tail concatemers contain multiple copies of the genome that must undergo cleavage to produce a unit-length viral genome. This is achieved by the pUL15/pUL28/pUL33 terminase complex, which recognizes a specific sequence on the concatemeric DNA and cleaves it to generate the free end for packaging initiation. After that, the pUL15/pUL28/pUL33 terminase complex hydrolyzes ATP to translocate negatively charged DNA into a procapsid through a dodecameric portal, forming the mature DNA-containing capsid. Successful encapsidation of HSV DNA also requires other four proteins, including UL6, UL17, UL25, and UL32. The newly synthesized mature naked nucleocapsids travel through the nuclear membrane via an envelopment-de-envelopment process. They migrate to Golgi-derived vesicle to acquire tegument and envelope, which are subsequently released from the host cell nucleus in a budding manner.
3. HSV Infection
HSV infection is mainly mediated through contact and can show two forms in the human body: latent infection and lytic infection. During primary infection, HSV infects epithelial cells in the mucosa or skin and then enters neurons of the peripheral nervous system (PNS) that interface with the epithelium after a period of replication. After entering neurons, viral nucleocapsids travel along microtubules to neuronal cell bodies. Subsequently, viral genomes enter neuronal nuclei, establishing a lifelong latent infection [6]. These is restricted gene expression and no generation of viral particles during latent infection. However, the appropriate stimuli such as stress or decreased immunity can activate the latent HSV, leading to the virus migration to epithelial cells and reactivation, which is the main cause of HSV repeated HSV infection [7].
HSV infection of susceptible non-neuronal cells usually causes lytic replication. Lytic replication involves a coordinated expression of viral genes, resulting in the production of infectious viruses.
4. HSV Transmission
HSV-1 mainly causes skin, mucous membranes, and central nervous system infection in the body above the waist, usually through the oral cavity, contaminated hands and droplets. HSV-1 infection is most common in children, and often causes oral gingival and stomatitis, most of which are recessive infections. It may also be sexually transmitted, including contact with saliva, such as kissing and mouth-to-genital contact (oral sex).
HSV-2 affects the body below the waist and is mostly transmitted through sexual activities, mainly genital skin and mucous membrane infection and neonatal infection, causing genital herpes. HSV-2 is periodically shed in the human genital tract, most often asymptomatically. Most sexual transmissions occur during periods of asymptomatic shedding [8]. However, recent studies have shown that both HSV-1 and HSV-2 play an important role in the pathogens that cause genital herpes.
Both HSV-1 and HSV-2 may also be transmitted vertically during childbirth. However, the risk of infection transmission is minimal if the mother has no symptoms or exposed blisters during delivery. The risk is considerable when the mother is infected with the virus for the first time during late pregnancy [9].
5. Immune Response to HSV Infection
The immune response against HSV is complex and involves a delicate interaction between innate and adaptive immune mechanisms [10]. The innate immune response is the first line of defense against incoming virus and plays a crucial role in the early control of infection and spread. Consequently, the generation of type I interferon (IFN), primarily consisting of IFNα and IFNβ, has been associated with defense against illness in both murine models and human investigations. Moreover, diverse cell types have been shown to contribute to the innate immune responses against HSV in vivo. Among these, natural killer (NK) cells play a pivotal role in anti-HSV immunity through cytokine production, recognition, and elimination of virally infected cells. Additionally, plasmacytoid dendritic cells (pDCs) are primarily involved in type I IFN production in vivo.
The adaptive immune response has demonstrated significant roles in disease progression, latency, and the control of virus spread.
References
[1] Weed D. J., Nicola A. V., Weed, Nicola (2017). Herpes simplex virus Membrane Fusion [J]. Adv. Anat. Embryol. Cell Biol. 223, 29–47.
[2] Sathiyamoorthy K., Chen J., Longnecker R., Jardetzky T. S. (2017). The COMPLEXity in herpesvirus entry [J]. Curr. Opin. Virol. 24, 97–104.
[3] Dohner, K., A. Wolfstein, U. Prank, C. Echeverri, D. Dujardin, R. Vallee, and B. Sodeik. 2002. Function of dynein and dynactin in herpes simplex virus capsid transport [J]. Mol. Biol. Cell 132795-2809.
[4] Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus [J]. J. Cell Biol. 1361007-1021.
[5] Luxton, G. W. G., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. From the cover: targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins [J]. Proc. Natl. Acad. Sci. 1025832-5837.
[6] Roizman B., Sears A.E. An inquiry into the mechanisms of herpes simplex virus latency [J]. Annu. Rev. Microbiol. 1987;41:543–571.
[7] Cliffe A.R., Wilson A.C. Restarting Lytic Gene Transcription at the Onset of Herpes Simplex Virus Reactivation [J]. J. Virol. 2017;91:e01419-16.
[8] Schiffer JT1, Mayer BT, Fong Y, Swan DA, Wald A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding [J]. PJ R Soc Interface. 2014 Mar 26; 11(95).
[9] Kimberlin DW. Herpes simplex virus infections of the newborn [J]. PSemin Perinatol. 2007 Feb; 31(1):19-25.
[10] Chew, T., Taylor, K. E., & Mossman, K. L. (2009). Innate and Adaptive Immune Responses to Herpes Simplex Virus [J]. Viruses, 1(3), 979-1002.