West Nile virus
West Nile virus (WNV) is a member of the family Flaviviridae, specifically from the genus Flavivirus, which also contains the Zika virus, dengue virus, and yellow fever virus. It is a mosquito-borne arbovirus first discovered in 1937 in the West Nile district of Uganda.
West Nile virus is primarily transmitted by mosquitoes, mostly species of Culex. The primary hosts of WNV are birds, but it can also affect mammals and reptiles [1]. People who get WNV usually have no symptoms or mild symptoms, up to 1% of those who develop serious and potentially fatal complications.
1. Structure of West Nile Virus
WNV is a positive-sense, single-stranded (SS) RNA virus. It is an enveloped virus with icosahedral symmetry [3] comprising three structural proteins that form the capsid (C), premembrane (PrM/M), envelope (E), and seven nonstructural proteins including NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 [2].
The WNV possesses an about 11kb long, single-stranded, positive sensed RNA genome, which is translated into a polyprotein that is subsequently cleaved into 10 functional proteins by both host and viral proteases. The structural proteins assemble virion particles and mediate host receptor binding and viral entry into host cells. Meanwhile, nonstructural proteins participate in viral genomic RNA replication and the regulation of host immune responses [4].
| WMV Proteins |
Function |
Products |
| Envelope (E) |
Receptor binding, viral attachment, and entry into the cell through membrane fusion [4] |
/ |
| Membrane protein (M) |
Structural protein that is involved in the formation of the viral envelope |
/ |
| Capsid (C) |
Package RNA into the developing viruses |
/ |
| Genome polyprotein |
Cleaved by virus and host proteases into separate viral proteins such as protein M and glycoprotein E |
Recombinant WNV Genome polyprotein |
| Non-structural Protein 1 (NS1) |
Plays a role in viral replication and immune evasion |
/ |
| Non-structural Protein 2A (NS2A) |
Involved in viral replication |
/ |
| Non-structural Protein 2B (NS2B) |
/ |
| Non-structural Protein 3 (NS3) |
Contains protease and helicase activities, essential for viral replication |
/ |
| Non-structural Protein 4A (NS4A) |
Involved in viral replication and modulating host cell responses |
/ |
| Non-structural Protein 4B (NS4B) |
/ |
| Non-structural Protein 5 (NS5) |
Contains the RNA-dependent RNA polymerase (RdRp) necessary for viral RNA replication |
/ |
2. West Nile Virus Infection
West Nile encephalitis is an infection of the brain that is caused by the West Nile virus. First identified in Uganda in 1937, the virus is commonly found in Africa, Canada, Europe, West Asia, and the Middle East.
- The majority (80%) of people infected with West Nile virus will experience no symptoms.
- About 20% people who are infected will experience flu-like symptoms including fever, headache, nausea, muscle pain, and swollen lymph glands.
- Other symptoms may include a stiff neck, rash, sleepiness or disorientation.
- If the virus crosses the blood-brain barrier, however, it can cause life-threatening conditions. About 1% of patients develop complications in the central nervous system (CNS) that affect the brain and spine, usually encephalitis, meningitis, or meningo-encephalitis.
- Individuals aged above 50 face a higher risk of experiencing severe illness, with approximately 10% of neurological infections resulting in fatality.
3. West Nile Virus Life Cycle
WNV's life cycle is maintained through its transmission between birds and mosquitoes. Birds can act as a reservoir and amplifying host of WNV. When a mosquito bites an infected bird, the virus enters the mosquito's bloodstream and eventually moves into its salivary glands. Once being bitten, the infected mosquito inoculates the virus into the host's skin.
WNV, akin to other flaviviruses, enters host cells through clathrin-mediated endocytosis with the assistance of various host factors. Subsequently, it releases its genome from the endosome into the cytoplasm following acidification-induced membrane fusion.
The viral RNA genome is transported through the cytosol to the endoplasmic reticulum (ER) using the host cellular machinery. In the ER, the viral positive-sense RNA is transcribed into negative-sense RNA for the replication of the progeny viral genome RNA. The synthesized viral RNA is translated into structural and non-structural proteins for the assembly of progeny viruses.
The structural proteins construct the nucleocapsid on ER membranes and then bud into the cytoplasm through the Golgi network. The progeny virus is transported to the cell surface in an exocytic vesicle and undergoes maturation upon cleavage of the prM by cellular enzymes, leading to the release of mature virions [7].
WNV can replicate in various cells including neutrophils, macrophages, and keratinocytes. Following spread, the virus undergoes an amplification phase, disseminating to visceral organs, and potentially progressing to the neuroinvasive phase, affecting the central nervous system (CNS).
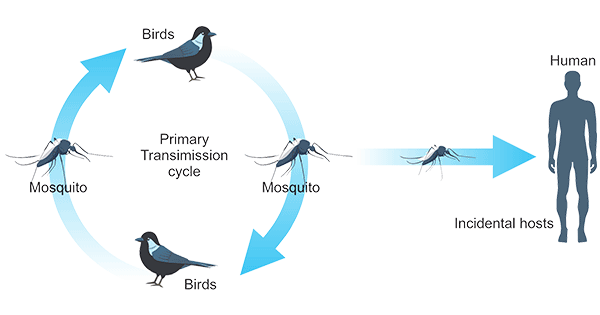
Figure 1. West Nile virus transmission cycle
4. West Nile Virus Transmission
WNV is transmitted to humans via the bite of an infected mosquito. Direct human-to-human transmission initially was believed to be caused only by occupational exposure, such as in a laboratory setting [5], or conjunctival exposure to infected blood [6]. The US outbreak identified additional transmission methods:
- Blood transfusions
- Organ transplants
- Pregnancy: An infected mother can infect her fetus, but the risk is very low.
- Breast-feeding: There is a very small chance of passing on the virus through breast milk. So that the Centers for Disease Control and Prevention (CDC) advise mothers to continue breast-feeding.
- Percutaneous and aerosol infections: These two infection ways have been reported in laboratory personnel.
5. Protective Immune Response to West Nile Virus
The protective immune response to WNV involves a coordinated effort among the innate and adaptive immune systems as well as the complement system, working together to recognize and eliminate the virus.
5.1 Innate Immunity
Recognition of WNV is initiated by pattern recognition receptors (PRRs) on the surface of cells, including TLRs. WNV can be recognized by TLR3 due to its ssRNA making dsRNA during genomic replication [8]. Infected cells produce interferons (IFNs) such as IFN-α and IFN-β as part of the antiviral response. IFNs signal neighboring cells to enter an antiviral state, inhibiting viral replication [9]. NK cells play a role in the early control of viral infections. They can directly kill infected cells and contribute to the modulation of the adaptive immune response.
5.2 Complement
The complement system, comprising interconnected proteins, defends against WNV through various pathways. Activation, involving classical, lectin, and alternative pathways, leads to the formation of the membrane attack complex (MAC). Genetic deficiencies in complement components increase susceptibility to WNV infection. Mannose-binding lectin (MBL) recognizes N-linked glycans on the structural proteins of WNV, contributing to neutralization through a C3- and C4-dependent mechanism that involves both the canonical and bypass lectin activation pathways [10]. WNV NS1 protein attenuates complement activation, and C1q enhances the potency of antibodies against WNV by regulating the stoichiometric requirements for neutralization [11].
5.3 Adaptive Immunity
Adaptive immunity against WNV involves B-cell and T-cell responses. B cells are responsible for producing antibodies that specifically recognize and neutralize WNV. These antibodies can inhibit viral entry and promote the clearance of circulating virus particles. Antibodies and B cells play a crucial role in defending against WNV infection, as evidenced by increased serum and CNS viral burdens and susceptibility to lethal infection in mice lacking B cells and antibodies [12]. The humoral immune response, mediated by antibodies, contributes to the prevention of WNV spread within the host. During WMV infection, early anti-WNV IgM limits viremia and propagation into the central nervous system. T cells, including both CD4+ helper T cells and CD8+ cytotoxic T cells, play a critical role in the cellular immune response against WNV. Helper T cells coordinate the immune response by assisting B cells in antibody production and activating other immune cells. Cytotoxic T cells directly target and kill virus-infected cells, limiting viral replication.
References
[1] Mackenzie, John S, et al. Emerging Flaviviruses: The Spread and Resurgence of Japanese Encephalitis, West Nile and Dengue Viruses [J]. Nat Med, 10, S98-109.
[2] Mazeaud, C.; Freppel, W.; Chatel-Chaix, L. The Multiples Fates of the Flavivirus RNA Genome During Pathogenesis [J]. Front. Genet. 2018, 9, 595.
[3] Suchetana Mukhopadhyay, Bong-Suk Kim, Paul R Chipman, et al. Structure of West Nile Virus [J]. Science, 302 (5643), 248 2003 Oct 10.
[4] Ryuta Kanai, Kalipada Kar, Karen Anthony, et al. Crystal Structure of West Nile Virus Envelope Glycoprotein Reveals Viral Surface Epitopes [J]. J Virol, 80 (22), 11000-8 Nov 2006.
[5] Centers for Disease Control and Prevention (CDC) (2002). Laboratory-acquired West Nile virus infections—United States, 2002 [J]. MMWR Morb. Mortal. Wkly. Rep. 51(50): 1133–5.
[6] Kevin Fonseca, Gerry D. Prince, Jeff Bratvold, et al. West Nile Virus Infection and Conjunctival Exposure [J]. Emerg Infect Dis. 2005 Oct; 11(10): 1648–1649.
[7] Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: Biology, Transmission, and Human Infection [J]. Clin. Microbiol. Rev. 2012, 25, 635–648.
[8] Town, T.; Jeng, D.; Alexopoulou, L.; Tan, J.; Flavell, R.A. Microglia recognize double-stranded RNA via TLR3 [J]. J. Immunol. 2006, 176, 3804–3812.
[9] Samuel, M. A., and Diamond, M. S. (2005). Alpha/Beta interferon protects against lethal West Nile Virus infection by restricting cellular tropism and enhancing neuronal survival [J]. J. Virol. 79, 13350–13361.
[10] Fuchs, A.; Lin, T.-Y.; Beasley, et al. Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin [J]. Cell Host Microbe 2010, 8, 186–195.
[11] Mehlhop, E.; Nelson, S.; et al. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus [J]. Cell Host Microbe 2009, 6, 381–391.
[12] Diamond, M.S.; Shrestha, B.; et al. B Cells and Antibody Play Critical Roles in the Immediate Defense of Disseminated Infection by West Nile Encephalitis Virus [J]. J. Virol. 2003, 77, 2578–2586.


