Rabies Virus
Rabies virus (RABV), belonging to the Lyssavirus genus within the Rhabdoviridae family, is a neurotropic virus that causes lethal encephalitis in warm-blooded animals. Globally, dogs are the most important vector of RABV. It is transmitted to humans from infected animals, primarily domestic dogs, through their saliva by biting or scratching. Rapid administration of rabies vaccine and human rabies immune globulin post-exposure is crucial to prevent rabies. However, there are no specific antiviral drugs that directly target the rabies virus.
1. The Structure of Rabies Virus
RABV exhibits a bullet-shaped enveloped structure, about 180 nm long and 75 nm diameters. Outermost, RABV is coated with spike-like glycoproteins (G) which are embedded in the bilayer lipid envelope. The matrix protein (M) is located on the inner viral envelope, inside which the viral nucleoprotein (N) tightly binds the viral RNA to form the nucleocapsid core. The spirally symmetrical nucleocapsid core, along with the phosphoprotein (P) and large transcriptase protein (L) constructs the viral nucleocapsid (RNP).
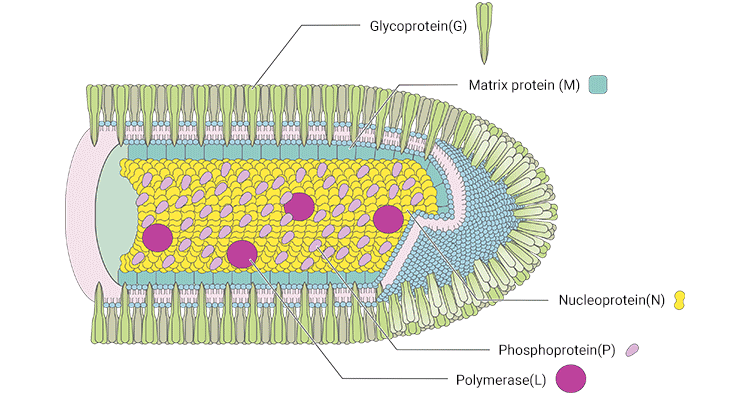
Figure 1. The structure of RABV
*This diagram is derived from the publication published on Annu Rev Virol [2]
RABV possesses a nonsegmented, single-stranded (SS), negative-sense RNA genome, about 11.9 kb [1]. The RABV's RNA genome encodes five structural proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and an RNA-directed RNA polymerase (L) [3].
N protein encapsulates the RNA genome, forming the ribonucleoprotein (RNP) complex [4]. The RNP is condensed into a helical nucleocapsid (NC) with L protein and P protein. P protein is a noncatalytic cofactor for the polymerase L. M protein is involved in budding, apoptosis, and intercellular membrane redistribution. G protein, which is trimeric and interacts at its cytoplasmic side with M protein, is the only protein exposed on the surface of the rhabdovirus envelope and is the sole ligand for the cellular receptor. G protein is involved in viral attachment and entry into host cells.
2. Rabies Virus Infection and Life Cycle
RABV infection initiates with the virus attaching to the cell surface and penetrating the cell through an endosomal transport pathway. After entering the host cell, the virus completes the synthesis of the structural proteins and RNA, which are essential for the assembly of progeny virus. The progeny viruses are released outside the cell by budding.
2.1 Viral Entry
RABV enters host cells through clathrin-mediated endocytosis. The virus binds to host cell membranes through the G protein and enters the cytoplasm through fusion. Following internalization, the viral G facilitates low pH-dependent fusion with the endosomal membrane, resulting in the uncoating of the virus and subsequent release of the helical nucleocapsid (NC) from the ribonucleoprotein (RNP).
2.2 Viral Replication
The five genes (N, P, M, G, and L) of the viral genomic RNA in the nucleocapsid are transcribed into five complementary monocistronic mRNAs by using the virion-associated RNA-dependent RNA polymerase. Each synthesized mRNA is subsequently translated into corresponding proteins, including N, P, M, G, and L proteins, which are involved in the assembly of progeny virus. After the synthesis of viral proteins, the genomic RNA replication process advances, producing full-length positive-stranded RNA, which serves as a template for the generation of progeny negative-stranded RNA.
2.3 Viral assembly and Release
The progeny RNA is encapsidated by the N-P complex, which incorporates the L protein to form the progeny RNP core. The M protein binds to the RNP core, condensing it into skeleton structures. These structures interact with trimeric G proteins anchored in the plasma membrane, assembling into virus particles. The progeny virions bud from the infected cells' plasma membrane into the nearby extracellular or interstitial space.
3. The Transmission of Rabies Virus to Animal or Humans
Rabies infection is caused by the rabies virus. The virus is spread through the saliva of infected animals. Infected animals can spread the virus by biting another animal or a person. Any mammal can transmit the rabies virus. The animals most likely to transmit the rabies virus to people include cat, cow, dog, ferret, goat, horse, bat, beaver, coyote, fox, monkey, raccoon, skunk, and woodchuck.
In rare cases, rabies virus can be transmitted when infected saliva gets into an open wound or the mucous membranes, such as the mouth or eyes. This could occur if an infected animal were to lick an open cut on your skin.
4. Pathogenesis of Rabies Virus
Accumulating evidence have proved that the pathogenesis of rabies virus can be divided into three stages during the incubation period and the onset period without viremia.
4.1 Breeding period in local tissues
After the RABV invades from the bite site, it accumulates and reproduces at the nerve fibers of the striated muscle spindle receptor of the wound, and then invades nearby peripheral nerves. The interval from a local wound to invasion of peripheral nerves is generally within 3 days, and some people think that the virus can stay in the invasion site for 2 weeks or even longer.
4.2 Invasion of the central nervous system
The RABV spreads centripetally along the axonal plasma of the peripheral nerve at a speed of about 3 mm/h. After arriving at dorsal root ganglion, the virus multiplies in it, and then invades the spinal cord and the entire central nervous system, mainly invading neurons in the brain and cerebellum.
4.3 Period of diffusion to various organs
The virus spreads centrifugally from the central nervous system to the peripheral nerves, invading various tissues and organs, especially the salivary nucleus, glossopharyngeal nucleus, and hypoglossal nucleus.
5. Recent Research and Advances
Recent advancements in rabies research have addressed various facets of the disease. Importation risks have been underscored by reported cases of canine rabies virus variants in dogs imported into the United States from high-risk countries like India, Iraq, and Egypt [5]. Insights into the biology of RABV have been expanded with the identification of key host factors crucial for RABV replication [6]. Diagnostic progress includes the development of a rapid and sensitive reverse transcription recombinase polymerase amplification assay for detecting rabies virus, suitable for low-resource settings [7].
A novel recombinant vaccine (ChAd68-Gp) utilizing a chimpanzee adenoviral vector has shown promising immune responses and protection in beagle dogs [8]. Advancements in mRNA vaccines encoding the main rabies virus antigen (RABV-G) have also been highlighted [9]. Evaluation of vaccination regimens and the necessity of specific visits has been investigated, with individuals exhibiting protective RABV neutralizing antibody titers [10].
A study on common vampire bats in Mexico revealed mortality events, emphasizing long incubation periods and changes in neutralizing antibody titers post-infection [11]. Additionally, a Knowledge, Attitude, and Practice (KAP) study in Uganda provided valuable insights into the prevalence of rabies and characterized Rabies virus strains [12]. These collective efforts contribute significantly to our understanding of rabies, spanning importation risks, diagnostics, vaccines, and epidemiological studies.
Rabies Research Associated Antibodies
| Product Name |
Species Reactivity |
Tested Applications |
Code |
| C Antibody |
Rabies virus |
ELISA, WB |
CSB-PA323345LA01RAI |
| G Antibody |
Rabies virus |
ELISA, WB |
CSB-PA14899A0Rb |
| G Antibody |
Rabies virus |
ELISA |
CSB-PA389005LA01RIE |
| M Monoclonal Antibody |
Rabies virus |
ELISA, WB |
CSB-MA322192A0m |
| M Antibody |
Rabies virus |
ELISA, WB |
CSB-PA322192LA01RAI |
| N Antibody |
Rabies virus |
ELISA |
CSB-PA321352LA01RAI |
| N Antibody |
Rabies virus |
ELISA |
CSB-PA321352YA01RAI |
| P Antibody |
Rabies virus |
ELISA, WB |
CSB-PA303154LA01RAH |
Rabies Research Associated ELISA Kits
References:
[1] Kuzmin IV, Tordo N. 2012. Genus Lyssavirus. In Rhabdoviruses: Molecular Taxonomy, Evolution, Genomics, Ecology, Host-Vector Interactions, Cytopathology and Control, ed. RG Dietzgen, IV Kuzmin, pp. 37–58. Norfolk, UK: Caister Acad.
[2] Benjamin M. Davis, Glenn F. Rall, et al. Everything You Always Wanted to Know About Rabies Virus (But Were Afraid to Ask) [J]. Annu Rev Virol. 2015.
[3] Dietzgen RG, Kuzmin IV. 2012. Taxonomy of rhabdoviruses. In Rhabdoviruses: Molecular Taxonomy, Evolution, Genomics, Ecology, Host-Vector Interactions, Cytopathology and Control, ed. RG Dietzgen, IV Kuzmin, pp. 13–22. Norfolk, UK: Caister Acad.
[4] Rahmeh AA, Schenk AD, et al. Molecular architecture of the vesicular stomatitis virus RNA polymerase [J]. PNAS. 2010, 107:20075–80.
[5] Yonette Hercules, Nelva Bryant, R. Wallace, et al. "Rabies in A Dog Imported from Egypt — Connecticut, 2017", MORBIDITY AND MORTALITY WEEKLY REPORT, 2018.
[6] Benoit Besson, Seonhee Kim, Taehee Kim, et al. "Kinome-Wide RNA Interference Screening Identifies Mitogen-Activated Protein Kinases And Phosphatidylinositol Metabolism As Key Factors For Rabies Virus Infection", MSPHERE, 2019.
[7] Jessica Coertse, Jacqueline Weyer, Louis H Nel, et al. "Reverse Transcription Recombinase Polymerase Amplification Assay for Rapid Detection of Canine Associated Rabies Virus in Africa", PLOS ONE, 2019.
[8] Xiang Wang, Zihao Fang, Jun Xiong, et al. "A Chimpanzee Adenoviral Vector-based Rabies Vaccine Protects Beagle Dogs From Lethal Rabies Virus Challenge", VIROLOGY, 2019.
[9] Nicole Armbruster, Edith Jasny, Benjamin Petsch, "Advances In RNA Vaccines For Preventive Indications: A Case Study Of A Vaccine Against Rabies", VACCINES, 2019.
[10] Tineke Cantaert, Laurence Borand, Lauriane Kergoat, et al. "A 1-week Intradermal Dose-sparing Regimen For Rabies Post-exposure Prophylaxis (RESIST-2): An Observational Cohort Study", THE LANCET. INFECTIOUS DISEASES, 2019.
[11] Elsa M Cárdenas-Canales, Crystal M Gigante, et al. "Clinical Presentation And Serologic Response During A Rabies Epizootic In Captive Common Vampire Bats (Desmodus Rotundus)", TROPICAL MEDICINE AND INFECTIOUS DISEASE, 2020.
[12] Michael Omodo, Meriadeg Ar Gouilh, Frank Norbert Mwiine, et al. "Rabies In Uganda: Rabies Knowledge, Attitude And Practice And Molecular Characterization Of Circulating Virus Strains", BMC INFECTIOUS DISEASES, 2020.


