Influenza Virus
Influenza viruses belong to the family Orthomyxoviridae, a family that represents enveloped viruses, the genome of which comprises segmented negative, single-strand RNA segments. People have had flu for thousands of years. It is speculated that humans may contract the flu at the beginning of domesticating animals. The development of agriculture and the establishment of permanent settlements provided numerous potential hosts that can cause epidemics. The word "flu" came from an Italian in the mid-17th century to describe a disease caused by miasma. The human influenza virus was not isolated until 1933.
Classification
Influenza viruses are mainly divided into three species: influenza A virus, influenza B virus, and influenza C virus. Only type A and B are clinically relevant to humans. Although both viruses can cause human infection, genera A typically leads to widespread illness and epidemics.
-
Influenza A virus
It was successfully isolated in 1933. Influenza A virus is commonly carried by animals such as birds, pigs, and is a pathogenic agent of zoonotic infections. Only influenza type A viruses are known to have caused pandemics. Influenza A virus undergoes an antigenic shift, which is a sudden change in antigenicity resulting from genetic recombination between viral strains, producing a new virus subtype. Based on the properties of the surface proteins hemagglutinin (H) and neuraminidase (N), influenza A viruses are subclassified and named by combining the H and N numbers, such as A(H1N1), A(H3N2).
-
Influenza B virus
Type B was obtained in 1940. It is only found in humans and usually causes milder symptoms than type A virus. Although it can trigger seasonal illness and human epidemics, influenza B virus has not caused a pandemic. Influenza B viruses are further classified into two lineages: B/Yamagata and B/Victoria.
-
Influenza C virus
Type C infections generally cause mild illness and are not thought to cause human flu epidemics.
Structure
Influenza A and B viruses are virtually indistinguishable by electron microscopy. They are typically spherical with a diameter of about 100 nm, but sometimes are filamentous with a length usually exceeding 300 nm. The outermost layer of influenza virus particles is the host cell-deriving envelope. The envelope is embedded with glycoprotein spikes of hemagglutinin (HA) and neuraminidase (NA), protruding from the envelope. Both HA and NA determine the subtypes of the influenza virus. The ratio of HA to NA is about 4:1. Less matrix ion channels M2 spans the lipid envelope. The matrix protein M1 is beneath the envelope and encapsulates the core of the virion.
Inward the virion is the viral genome composed of 8 pieces of single-stranded RNA (only type A and B). The RNA, coated with nucleoprotein and heterotrimeric RNA-dependent RNA polymerase (three subunits: PB1, PB2, and PA), is assembled into a helical ribonucleoprotein (RNP) complex. Nuclear export protein (NEP) is also found in the interior of the virion.
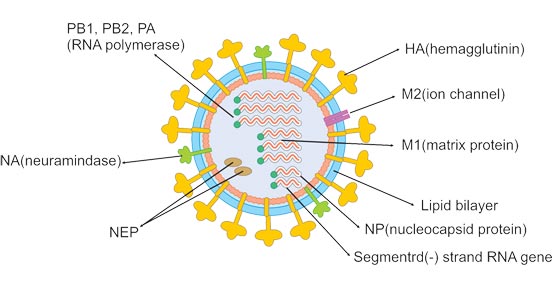
Figure 1.
| Target |
Protein |
Description |
| HA |
Hemagglutinin |
The HA protein is processed into HA1 and HA2 by serine proteases during viral replication, which gives virus infectivity. The HA2 is involved in the fusion of viral envelope with host cell membrane, while the HA1 contains the receptor binding and antigenic sites. It is also an antigen that can be recognized by the immune system. |
| NA |
Neuraminidase |
It is capable of destroying receptor, cleaving terminal sialic acid residues from cell-surface glycoproteins and gangliosides to release offspring virus from the host cell. Additionally, the NA also removes sialic acid residues from the virus envelope, blocking the aggregation of viral particles to enhance infectivity. |
| M1 |
matrix protein M1 |
M1 interacts with both viral RNA and nucleoprotein, bringing them together within the RNP complex. It also associates with NEP, mediating the M1-RNP export via nucleoporins into the cytoplasm. |
| M2 |
matrix ion channels M2 |
It is the target of the amantadine class of anti-influenza drugs, which inhibit ion channel activity and prevent virus from unshelling. As a surface protein, it can be a vaccine component. |
| NEP/NS |
Nuclear export protein |
NEP, together with M1, is involved in the translocation of M1-RNP into the cytoplasm. |
| NP |
Nucleoprotein |
Viral RNA is packaged with nucleoprotein into a helical ribonucleoprotein complex. |
| RNP |
Ribonucleoprotein |
The RNA, coated with nucleoprotein and heterotrimeric RNA-dependent RNA polymerase (three subunits: PB1, PB2, and PA), assembls into a helical ribonucleoprotein (RNP) complex. |
See All Related Proteins of Influenza-Virus
The Influenza Pandemic Event
In 1918-1920 Spanish Flu, more than 50 million people died of H1N1 influenza worldwide. About 1.5 to 2 million people died in the 1957-1958 Asian flu pandemic, which was caused by the H2N2 influenza A subtype. The H3N2 led to the 1968-1969 Hong Kong Flu, causing 1million deaths. The bird flu panic of 2006 was caused by a strain of Influenza A. The swine flu of 2009 caused a great deal of widespread sickness and fear. This was a new strain of influenza A called H1N1 and was carried by pigs but quickly spread through humans. The 2009 Swine Flu was spread to 208 countries and caused approximately 18000 deaths. The pandemic of H7N3 avian influenza in Mexico in late 2012 was the largest ever outbreak in poultry in North America, with 20 million birds killed. And influenza has become a typical seasonal flu and causes about 290000 to 650000 deaths annually around the world.
Transmission
Influenza is a typical seasonal diseases and frequently occurs in spring and winter. There are three transmission routes of influenza virus.
-
Through aerosol infection
The influenza virus is mainly transmitted by aerosol infection. For example, large droplets (larger 5 μm in diameter) produced when talking, coughing, or sneezing enters the mucous membrane through short-distance contact.
-
Through aerogenic transmission
Propagation may occur in tiny droplet cores (smaller 5 μm in diameter) that can stay in the air for longer periods.
-
Through direct contact
Direct contact with virus-contaminated surfaces (such as handshaking) and subsequent oral and nasal contact can cause transmission. Isolation and identification of virus
Related Proteins
| Uniprot No. |
Species |
Product Name |
| Q0HD60 |
H1N1 |
Recombinant Influenza A virus Hemagglutinin (HA), partial |
| P88838 |
H7N7 |
Recombinant Influenza A virus Neuraminidase (NA), partial |
| P69291 |
H3N2 |
Recombinant Influenza A virus Nucleoprotein (NP) |
| Q9IQ47 |
H3N2 |
Recombinant Influenza A virus Polymerase acidic protein (PA), partial |
| Q0HD54 |
H1N1 |
Recombinant Influenza A virus Non-structural protein 1 (NS) |
| A4GCL0 |
H1N1 |
Recombinant Influenza A virus Matrix protein 1 (M) |
| A4GCM0 |
H1N1 |
Recombinant Influenza A virus Matrix protein 2 (M), partial |
| P0C0U1 |
H1N1 |
Recombinant Influenza A virus Protein PB1-F2 (PB1) |
| P12445 |
H7N1 |
Recombinant Influenza A virus Polymerase basic protein 2 (PB2), partial |
| A8C8X2 |
H1N1 |
Recombinant Influenza A virus Putative protein PB1-F2 (PB1-F2) |
| P04665 |
Influenza B virus |
Recombinant Influenza B virus Nucleoprotein (NP) |
| P03502 |
Influenza B virus |
Recombinant Influenza B virus Non-structural protein 1 (NS) |
| P68760 |
Influenza B virus |
Recombinant Influenza B virus Hemagglutinin (HA) |
| P67907 |
Influenza B virus |
Recombinant Influenza B virus Neuraminidase (NA), partial |
| P13875 |
Influenza B virus |
Recombinant Influenza B virus Polymerase basic protein 2 (PB2), partial |
| P13880 |
Influenza B virus |
Recombinant Influenza B virus Matrix protein 1 (M) |
| P11136 |
Influenza B virus |
Recombinant Influenza B virus Polymerase acidic protein (PA), partial |
| P13872 |
Influenza B viru |
Recombinant Influenza B virus RNA-directed RNA polymerase catalytic subunit (PB1), partial |
| P13881 |
Influenza B virus |
Recombinant Influenza B virus Matrix protein 2(M), partial |

