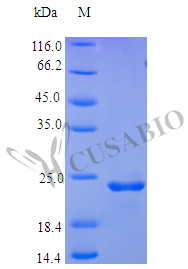
Recombinant Human Oncostatin-M protein (OSM) is produced in E. coli and spans the 26-234 amino acid region, providing a partial, tag-free protein. This product achieves a purity level of over 97%, confirmed by SDS-PAGE analysis. It appears to be fully biologically active, with an ED50 of less than 2 ng/ml determined via a human TF-1 cell proliferation assay, indicating a specific activity of greater than 5.0 × 10^5 IU/mg. Endotoxin levels are maintained below 1.0 EU/µg as per LAL testing.
Oncostatin-M is a cytokine that belongs to the interleukin-6 family. It plays what appears to be a significant role in cell growth regulation, differentiation, and inflammation. This protein gets involved in various signaling pathways and has implications in research focusing on cell proliferation and immune responses. Researchers often turn to this protein as a tool for exploring cellular processes and understanding its influence across different biological contexts.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Cell Proliferation and Viability Assays
This recombinant OSM protein is confirmed to be highly biologically active (ED₅₀ < 2 ng/ml in TF-1 cells) and suitable for studying cytokine-induced proliferation. However, the partial sequence (26-234aa) may lack C-terminal domains present in full-length OSM that could affect some biological functions. The high specific activity (>5.0×10⁵ IU/mg) supports reliable dose-response studies, but researchers should validate that proliferation effects match those of full-length OSM in their specific cell models.
2. JAK-STAT Signaling Pathway Studies
The biologically active OSM is appropriate for JAK-STAT signaling studies, but the partial sequence may alter signaling kinetics or amplitude compared to full-length OSM. The tag-free design ensures authentic receptor engagement, but researchers should validate that STAT phosphorylation patterns and downstream gene expression changes match those induced by full-length OSM, particularly for pathways requiring complete receptor complex formation.
3. Cytokine Receptor Binding and Competition Studies
This OSM can be used for receptor binding studies, but the partial sequence (lacking the C-terminal 18 amino acids) may affect binding characteristics with OSM receptor complexes (OSMRβ/gp130). Binding assays should include comparison with full-length OSM to confirm that the truncation does not significantly alter receptor affinity or specificity. The high purity supports accurate measurements, but the partial nature may limit studies of certain receptor interactions.
4. Inflammatory Response Research Models
The protein is suitable for inflammatory models, but the partial sequence may not fully recapitulate native OSM's inflammatory functions. Researchers should validate that key inflammatory responses (e.g., acute phase protein induction, cytokine production) match those elicited by full-length OSM. The low endotoxin content ensures specific attribution of effects to OSM activity.
5. Antibody Development and Validation
This high-purity OSM serves as a good antigen, but antibodies generated against this partial sequence may not recognize epitopes in the missing C-terminal region. Antibodies should be validated against full-length OSM to ensure comprehensive epitope coverage. The confirmed bioactivity supports the development of function-blocking antibodies targeting the receptor-binding domains.
Final Recommendation & Action Plan
This recombinant human OSM partial protein (26-234aa) demonstrates excellent biological activity in TF-1 cell proliferation assays, making it suitable for most proposed applications with appropriate validation. However, the partial nature (lacking the C-terminal 18 amino acids of full-length OSM) requires caution. First, validate that key biological functions (signaling, receptor binding, inflammatory responses) match those of full-length OSM in your specific experimental systems. For cell-based assays, the high potency and low endotoxin support reliable results, but confirm that proliferation and signaling kinetics are comparable to full-length OSM. When developing antibodies, supplement with the full-length antigen to ensure recognition of all relevant epitopes. For receptor studies, the tag-free design is advantageous, but compare binding characteristics with full-length OSM to account for potential C-terminal domain contributions. While the E. coli expression produces a non-glycosylated protein, the demonstrated high bioactivity confirms proper folding for core OSM functions. Always include appropriate controls and consider using full-length OSM for validation of critical findings.