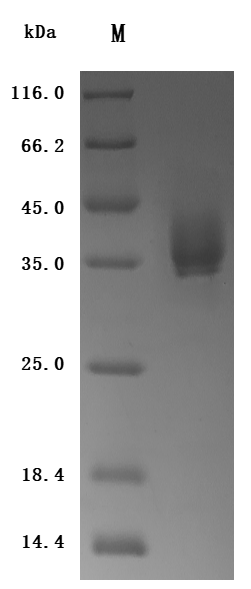
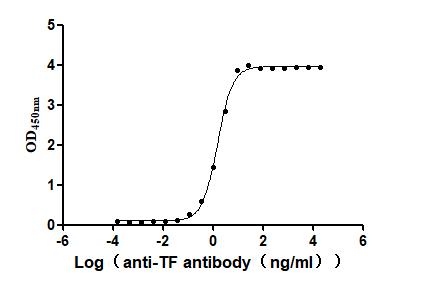
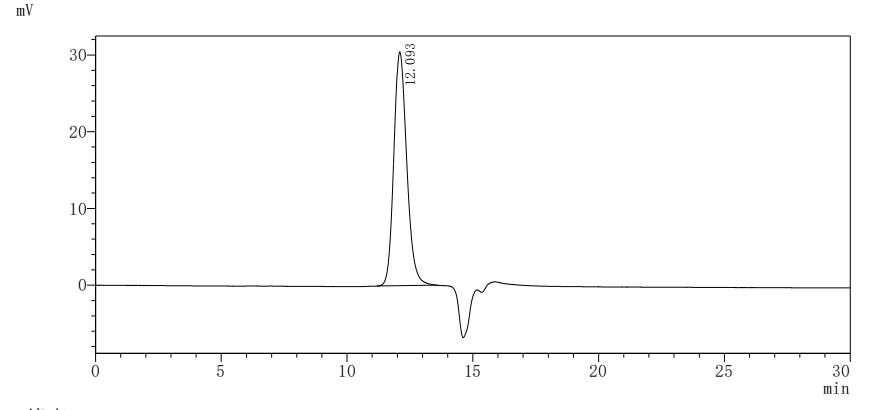
The generation for the recombinant human tissue factor (F3) involves gene amplification, plasmid creation, protein expression, purification, and characterization. Specific primers amplify the tissue factor region corresponding to amino acids 33-251, which is inserted into a plasmid containing a C-terminal 10xHis-tag. Mammalian cells are transfected with the plasmid, and after 24 hours, a selective antibiotic is used to identify the target protein-producing cells. The selected cells are cultured and induced to express the target proteins. The recombinant human tissue factor is released through cell lysis and purified from the supernatant using Ni-NTA affinity chromatography. The purified tissue factor protein achieves >95% purity (SDS-PAGE and SEC-HPLC) and <1.0 EU/μg endotoxin levels (LAL). ELISA confirms the functional binding of this recombinant tissue factor to the TF recombinant antibody (CSB-RA007928MA2HU) with an EC50 of 1.434-1.635 ng/mL.
Human tissue factor (TF), also known as thromboplastin, is a critical transmembrane glycoprotein that plays a pivotal role in the initiation of the extrinsic pathway of blood coagulation. It is primarily expressed in cells that are not typically in contact with blood, such as fibroblasts and endothelial cells, but can be induced by inflammatory cytokines, particularly in pathological conditions [1][4][10]. Upon vascular injury, TF binds to circulating factor VIIa (FVIIa), forming a complex that activates FIX and FX, leading to thrombin generation and subsequent clot formation [1][4][12]. TF also has significant roles beyond coagulation, influencing various pathophysiological processes including inflammation, angiogenesis, and tumor progression [2][9][12].
The signaling pathways activated by the TF-FVIIa complex are mediated through protease-activated receptors (PARs), specifically PAR1 and PAR2. These receptors are G-protein-coupled receptors that, when activated, can trigger a cascade of intracellular signaling events that contribute to cellular responses such as proliferation, migration, and survival [2][8]. The interaction of TF with FVIIa not only initiates coagulation but also modulates cellular behavior in non-hemostatic contexts, such as in cancer metastasis and inflammation [2][9][12]. The TF-FVIIa complex has been shown to enhance endothelial cell permeability and promote angiogenesis, which is crucial for tumor growth and metastasis [11][12].
Moreover, the regulation of TF activity is tightly controlled by tissue factor pathway inhibitor (TFPI), which serves as a natural anticoagulant. TFPI inhibits the TF-FVIIa complex and factor Xa, thereby preventing excessive coagulation and maintaining hemostatic balance [5][6][7]. The expression of TFPI can be modulated by various factors, including inflammatory cytokines, which can influence the overall coagulation response and the balance between pro-coagulant and anti-coagulant activities in the body [3][7]. Understanding the role of TF in coagulation and signaling pathways is crucial for developing therapeutic strategies for conditions such as thrombosis, cancer, and inflammatory diseases.
References:
[1] Adams, M., Thom, J., Hankey, G., Baker, R., Gilmore, G., Staton, J., … & Eikelboom, J. (2006). The tissue factor pathway in ischemic stroke. Blood Coagulation & Fibrinolysis, 17(7), 527-532. https://doi.org/10.1097/01.mbc.0000245294.41774.06
[2] Ahamed, J. and Ruf, W. (2004). Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. Journal of Biological Chemistry, 279(22), 23038-23044. https://doi.org/10.1074/jbc.m401376200
[3] Ahamed, J., Belting, M., & Ruf, W. (2005). Regulation of tissue factor–induced signaling by endogenous and recombinant tissue factor pathway inhibitor 1. Blood, 105(6), 2384-2391. https://doi.org/10.1182/blood-2004-09-3422
[4] Camerer, E., Huang, W., & Coughlin, S. (2000). Tissue factor- and factor x-dependent activation of protease-activated receptor 2 by factor viia. Proceedings of the National Academy of Sciences, 97(10), 5255-5260. https://doi.org/10.1073/pnas.97.10.5255
[5] Girard, T., Grunz, K., Lasky, N., Malone, J., & Broze, G. (2018). Re‐evaluation of mouse tissue factor pathway inhibitor and comparison of mouse and human tissue factor pathway inhibitor physiology. Journal of Thrombosis and Haemostasis, 16(11), 2246-2257. https://doi.org/10.1111/jth.14288
[6] Lizakowski, S., Zdrojewski, Z., Jagodziński, P., & Rutkowski, B. (2007). Plasma tissue factor and tissue factor pathway inhibitor in patients with primary glomerulonephritis. Scandinavian Journal of Urology and Nephrology, 41(3), 237-242. https://doi.org/10.1080/00365590601016511
[7] Mast, A. (2016). Tissue factor pathway inhibitor. Arteriosclerosis Thrombosis and Vascular Biology, 36(1), 9-14. https://doi.org/10.1161/atvbaha.115.305996
[8] Rao, L. and Pendurthi, U. (2005). Tissue factor–factor viia signaling. Arteriosclerosis Thrombosis and Vascular Biology, 25(1), 47-56. https://doi.org/10.1161/01.atv.0000151624.45775.13
[9] Riewald, M. and Ruf, W. (2001). Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proceedings of the National Academy of Sciences, 98(14), 7742-7747. https://doi.org/10.1073/pnas.141126698
[10] Sethi, A., Lees, D., Douthwaite, J., & Corder, R. (2005). Factor viia stimulates endothelin-1 synthesis in tnf-primed endothelial cells by activation of protease-activated receptor 2. Clinical Science, 108(3), 255-263. https://doi.org/10.1042/cs20040237
[11] Uusitalo‐Järvinen, H., Kurokawa, T., Mueller, B., Andrade‐Gordon, P., Friedlander, M., & Ruf, W. (2007). Role of protease activated receptor 1 and 2 signaling in hypoxia-induced angiogenesis. Arteriosclerosis Thrombosis and Vascular Biology, 27(6), 1456-1462. https://doi.org/10.1161/atvbaha.107.142539
[12] Versteeg, H. and Ruf, W. (2006). Emerging insights in tissue factor-dependent signaling events. Seminars in Thrombosis and Hemostasis, 32(01), 024-032. https://doi.org/10.1055/s-2006-933337