Parainfluenza Virus: Structure, Classification, Pathogenic Mechanism and Drug Research Progress
Human Parainfluenza Virus (HPIV) belongs to the family Paramyxoviridae. Also belonging to the Paramyxoviridae family are human mumps virus, measles virus, respiratory syncytial virus, and metapneumovirus.
HPIV is one of the major pathogens causing acute respiratory infections in infants, young children and adolescents, and can cause severe lower respiratory tract infections, especially in infants, young children, the elderly, and immunocompromised adults. HPIV is prevalent globally and is highly contagious and pathogenic, mainly through droplet and contact transmission. It is important to note that although HPIV has a similar name to the influenza virus and causes acute respiratory infections, they are completely different viruses and the influenza vaccine does not protect against HPIV infection.
Based on serological and genomic characteristics, parainfluenza can be classified into four serotypes ranging from HPIV1 to HPIV4, each of which may cause different types of respiratory illnesses, with symptoms ranging from mild colds to severe illnesses such as pneumonia and laryngitis. In the following paragraphs, we will discuss in detail the structure, classification, pathogenic mechanism and the current progress of drug research on parainfluenza viruses.
1. Structure of parainfluenza virus
Parainfluenza virus (PIV) is a single-stranded, negative-stranded RNA virus, characterised by polymorphism and encapsulated within an envelope consisting of lipids and glycoproteins ranging in diameter from 125 to 250 nm. The genome of PIV, a negative-sense RNA strand, follows a conserved order to encode six key essential proteins:
- Nucleocapsid protein (NP): This protein binds tightly to the viral RNA, forming a core structure that wraps around the RNA. It provides a template for the RNA-dependent RNA polymerase (composed of P and L proteins) to facilitate the viral transcription process.
- Phosphoprotein (P): Together with the L protein, it forms the RNA polymerase complex, which is involved in viral transcription.
- RNA polymerase (L): Together with the P protein, it forms the RNA polymerase complex that catalyses viral RNA synthesis.
- Matrix protein (M): Is a key driver for the assembly and outgrowth of viral particles at the host cell membrane. It is located in the inner layer of the viral envelope and works in conjunction with other structural proteins such as nucleocapsid proteins and RNA polymerase to aid in the formation and release of viral particles.
- Fusion Glycoprotein (F): This is a surface protein that mainly mediates the fusion process between the viral envelope and the host cell membrane. The F protein plays a crucial role in the invasion of the virus into the host cell.
- Haemagglutinin neuraminidase (HN) glycoprotein: Another surface protein that possesses not only erythrocyte agglutination activity, but also neuraminidase activity.HN proteins play an important role in the life cycle of viruses by attaching to sialic acid residues on the surface of the host epithelial cell and by cleaving these residues to facilitate the release of new virus particles from the cell.
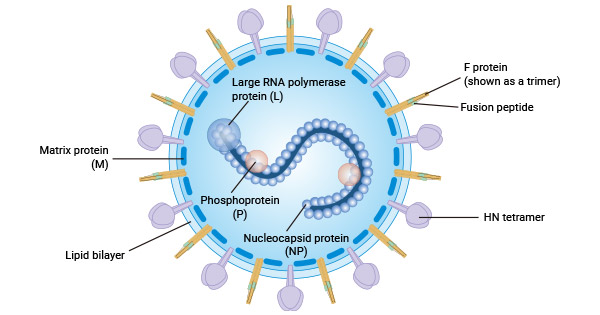
Figure. Structure of parainfluenza virus HPIV
2. Classification of parainfluenza viruses
Of the four virus types, HPIV-1 and HPIV-3 belong to the genus Respiratory Virus and HPIV-2 and HPIV-4 belong to the genus Mumps Virus.
- HPIV-1 mainly causes laryngotracheobronchitis (croup), a respiratory disease characterised by swelling of the vocal cords and other parts of the upper respiratory tract. Clinical manifestations include hoarseness, barking cough and laryngeal ringing, which are particularly common in children. Epidemiologically, HPIV-1 infection has a distinct seasonal pattern, usually with a high incidence in autumn and early winter.
- HPIV-2 is an important paediatric respiratory pathogen that encodes a V protein that inhibits type I interferon (IFN) induction and signalling by interacting with MDA5, and its virulence in non-human primates is important [1].
- HPIV-3 mainly affects children under 5 years of age and is usually associated with fine bronchitis and pneumonia, and its infection is widespread worldwide and peaks annually, especially in spring and summer [2].
- HPIV-4 can be divided into two antigenically distinct subtypes, HPIV-4a and HPIV-4b, which mainly affects the elderly, accounting for 12.24% of people over 65 years of age, and there is no clear seasonal pattern to its infection. In one study, HPIV-4 was detected in 5.93% of all acute respiratory infections (ARIs), the least detected type of the four HPIVs [3].
3. Pathogenesis Mechanisms of Parainfluenza Virus
3.1 Virus invasion and receptor binding
After entering the human body via droplet transmission, the parainfluenza virus first binds to specific receptors on the respiratory mucosa. These receptors are usually located on the surface of respiratory epithelial cells and provide a gateway for the virus to enter the cells. Once the virus binds to the receptor, fusion proteins on its envelope mediate the fusion of the viral envelope with the host cell membrane, resulting in the release of the viral nucleocapsid into the cytoplasm.
3.2 Viral replication and gene expression
Upon entering the cytoplasm, the RNA genome of the parainfluenza virus begins to replicate and is transcribed into mRNA, which is then translated into viral proteins. These viral proteins include new viral RNA polymerase, nuclear capsid proteins, envelope proteins (e.g., haemagglutinin-neuraminidase HN and fusion protein F), etc., which together are involved in the process of virus assembly and replication.
3.3 Cellular damage and immune response
Massive replication of parainfluenza viruses within cells leads to cellular damage and death. The expression of viral proteins may interfere with the normal physiological functions of host cells, such as interfering with protein synthesis and disrupting cell membrane integrity. At the same time, viral infection triggers host immune responses, including innate and adaptive immunity. Innate immune responses, such as the production and release of interferon, can inhibit viral replication, while adaptive immune responses clear virus-infected cells by producing specific antibodies and T cells.
3.4 Lesions and symptoms
Parainfluenza viruses primarily invade the superficial tissues of the respiratory mucosa and proliferate within the epithelial cells. This leads to congestion, oedema and increased secretion of the respiratory mucosa, which in turn leads to a series of respiratory symptoms such as fever, cough, nasal congestion and runny nose.
In infants, the elderly and immunodeficient adults, parainfluenza viruses may further invade the lower respiratory tract, causing serious illnesses such as bronchiolitis and pneumonia. These illnesses may lead to severe symptoms such as dyspnoea, shortness of breath, cyanosis, etc., and may even be life-threatening.
4. Drug development Progress for parainfluenza viruses
Currently, the treatment of parainfluenza virus infection relies mainly on antiviral drugs and symptomatic supportive therapy. Commonly used antiviral drugs include ribavirin, oseltamivir, and acyclovir. Ribavirin can be administered by nebulised inhalation, orally or intravenously, but it may be ineffective in the later stages of HPIV infection, particularly in the presence of respiratory failure. In addition, alpha-interferon and thymosin may be used to enhance immune function.
A number of novel antiviral agents are in development, such as the sialidase fusion protein DAS181, the HN inhibitor BCX2798, and the lipopeptide VIQKI, derived from the C-terminal heptapeptide repeat structural domain of the HPIV F protein.DAS181 has been approved for the treatment of HPIV-associated pneumonia following lung transplantation and haematopoietic stem cell transplantation in recipients, and has shown in phase II clinical trials an increased resistance to potential efficacy in severely immunocompromised patients with mild to moderate hypoxia.
Although no effective vaccine against HPIV is currently on the market, vaccine development is actively underway. Research has focused on live attenuated vaccines, subunit vaccines, and mRNA vaccines. Some vaccines against HPIV-3 have entered clinical trials, and early studies have shown that cold-adapted live attenuated HPIV-3 vaccines have good immunogenicity and protection.
5. CUSABIO Parainfluenza Virus Research Related Products
5.1 Parainfluenza virus Recombinant proteins
5.1 Parainfluenza Virus ELISA Kits
Reference:
[1] Identification of Human Parainfluenza Virus Type 2 (HPIV-2) V Protein Amino Acid Residues That Reduce Binding of V to MDA5 and Attenuate HPIV-2 Replication in Nonhuman Primates. Journal of Virology, 2011.
[2] Protective Antibodies Against Human Parainfluenza Virus Type 3(HPIV3) Infection. MAbs, 2021.
[3] Epidemiological Characteristics of Human Parainfluenza Viruses Infections-China, 2019-2023. Vital Surveillances.


