Respiratory Syncytial Virus (RSV)
Respiratory Syncytial Virus (RSV) is a non-segmented, single-stranded negative-sense RNA virus, belonging to the family Paramyxoviridae, within the genus Pneumovirus, and is named for its ability to fuse infected cells.
RSV can cause respiratory infections in infants, the elderly, and individuals with weakened immune systems worldwide. It is the leading cause of lower respiratory tract infections in children under 5 years old, leading to approximately 33 million cases of lower respiratory tract infections annually.
1. Structure of the RSV Virus
The RSV virion exhibits a variety of morphologies, including spherical, asymmetrical, and filamentous shapes, with diameters around 130 nanometers and lengths ranging from several hundred nanometers to over 10 micrometers.
The structural components of RSV primarily consist of the viral envelope and the internal ribonucleoprotein complex (RNP). The viral envelope is composed of glycoproteins such as the fusion protein (F), attachment protein (G), and small hydrophobic protein (SH). These glycoproteins play a crucial role in the virus's attachment, fusion, and infection processes. The G protein, which is the primary attachment protein of the virus, binds to host cell receptors, while the F protein facilitates the fusion of the viral envelope with the host's membrane. The ribonucleoprotein complex of RSV is made up of RNA and the nucleocapsid protein (N protein), which forms a helical structure within the virus particle, protecting and replicating the viral RNA [1].
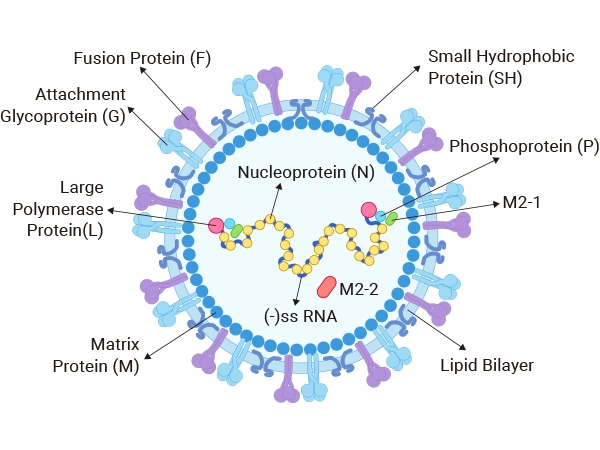
Figure 1. Structure of the respiratory syncytial virus
The genome of the RSV virus is composed of a single-stranded negative-sense RNA with 10 open reading frames (ORFs), with a total length of approximately 15.2 kb. It encodes 11 structural and non-structural proteins, each playing its own role in the virus's replication, assembly, and infection processes. The specific proteins are as follows:
| Protein Names |
Functional |
| Fusion Protein (F) |
Mediates the fusion of the virus with the host cell membrane, allowing the virus to enter the cell. It is an important antigenic site for the production of neutralizing antibodies and is a primary target for inducing immunogenicity and antiviral responses. |
| Attachment Protein (G) |
Responsible for attaching the virus to the host cell surface, initiating the infection process. The G protein is highly variable and is closely related to the determination of antigenic epitopes and the diversity of viral genes, playing a significant role in distinguishing between RSV A and B subtypes. |
| Small Hydrophobic Protein (SH) |
A transmembrane protein, its specific functions may involve the infection and replication processes of the virus, but it is less important compared to the F and G proteins. |
| Nucleoprotein (N) |
Binds to the viral RNA to form the nucleocapsid, protecting the virus's genetic material. |
| Phosphoprotein (P) |
Together with the N protein and L protein, it constitutes the viral replication complex, participating in the viral replication process. |
| RNA Polymerase Protein (L) |
Possesses RNA polymerase activity, responsible for the synthesis and replication of the virus's RNA. |
| Matrix Protein M1 (M) |
Located beneath the lipid envelope, it binds to the replication complex and participates in the assembly process of the virus. |
| Matrix Protein M2-1 (M2-1) |
Together with the M protein, it participates in the assembly and budding process of the virus, and may also have a role in regulating viral RNA synthesis. |
| Matrix Protein M2-2 (M2-2) |
Involved in the replication and transcription processes of the virus, potentially affecting the virus's replication efficiency by regulating the synthesis of viral RNA. |
| Non-structural Protein NS1 |
Its specific functions are not fully understood, but it may be involved in regulating the replication and infection processes of the virus. |
| Non-structural Protein NS2 |
Also has the function of regulating viral replication and infection processes, but the specific mechanisms are yet to be studied. |
2. Infection Mechanism of the RSV Virus
The infection mechanism of RSV involves several steps, including viral attachment, entry, replication, assembly, and release:
2.1 Viral Attachment:
RSV spreads through droplets and initially attaches to the upper respiratory tract epithelial cells of the host. The virus's surface G protein (a glycoprotein) recognizes and binds to specific receptors on the host cell surface. These receptors include heparan sulfate proteoglycans (HSPGs) and other potential receptor molecules.
2.2 Viral Entry
After attachment, the conformation of the virus's surface F protein (fusion protein) changes, mediating the fusion of the viral envelope with the host cell membrane, allowing the viral nucleocapsid to enter the host cell. In addition to membrane fusion, RSV can also enter cells through endocytosis. The virus is engulfed by the host cell through endocytosis, forming an endosome. Within the endosome, the acidic environment further activates the F protein, ultimately leading to the fusion of the viral envelope with the endosome membrane and releasing the viral nucleocapsid into the cytoplasm.
2.3 Viral Replication
Once the viral nucleocapsid enters the host cell, the RSV nucleocapsid disassembles, releasing the single-stranded negative-sense RNA genome. The viral RNA genome is recognized and transcribed into positive-sense mRNA by the virus's RNA-dependent RNA polymerase (composed of the L and P proteins). These mRNAs are then translated into viral proteins, including structural and non-structural proteins. The viral RNA polymerase is also responsible for synthesizing new negative-sense RNA genomes, which are packaged into new virus particles.
2.4 Viral Assembly and Release
The newly synthesized viral RNA combines with the N protein to form a new nucleocapsid. The new nucleocapsid moves near the host cell membrane, interacts with the M protein, recruits the G and F proteins on the membrane, and forms new virus particles. Mature virus particles are released into the extracellular environment through the budding process of the host cell membrane. During this process, the virus particles acquire a portion of the host cell membrane as their envelope.
2.5 Immune Response and Pathology
RSV infection triggers the host's innate and adaptive immune responses. The innate immune response includes the production of interferons and other inflammatory mediators, while the adaptive immune response involves the production of specific antibodies and T cell responses. RSV infection leads to damage of airway epithelial cells, infiltration of inflammatory cells, increased airway secretions, airway narrowing, and hyperresponsiveness. These pathological changes can cause symptoms such as coughing, wheezing, difficulty breathing, and in severe cases, may lead to bronchitis and pneumonia.
Understanding the infection mechanism of the RSV virus is crucial for developing effective prevention and treatment strategies. Currently, research on vaccines and antiviral drugs for RSV infection is making progress, aiming to mitigate the transmission and pathogenicity of RSV by intervening in these key steps.
3. Main Targets and Research Progress of RSV Antiviral Drugs
The research on RSV antiviral drugs primarily focuses on targets such as the F protein, RNA-dependent RNA polymerase (RdRp), host factors, and the deoxyguanosine nucleotide synthesis pathway. Research on these targets provides an essential foundation for the development of new anti-RSV drugs.
3.1 Fusion Protein (F Protein)
The F protein is a key factor for RSV entry into host cells, facilitating the process by mediating the fusion of the viral envelope with the host cell membrane. Therefore, inhibitors targeting the F protein are one of the research focuses. For instance, GS-5806 and BMS-433771 both inhibit virus entry into host cells by blocking F protein-mediated membrane fusion [2]. Additionally, RV521 is a small molecule inhibitor targeting the RSV-F protein that has shown efficacy in clinical trials by reducing viral load and improving disease severity [3].
3.2 RNA-Dependent RNA Polymerase (RdRp)
The RNA polymerase of RSV is another significant target for antiviral drugs. Inhibiting the virus's RNA replication process can effectively block the virus's life cycle. For example, structure-based design strategies have identified new allosteric binding sites on the RSV RdRp, leading to the development of small molecule inhibitors with antiviral activity [4].
3.3 Host Factors
In recent years, an increasing number of studies have begun to focus on antiviral drugs targeting host factors. For example, the nucleoprotein (N protein) and G protein are also important targets because they play a key role in the viral infection process [5]. Moreover, nucleolin has been discovered as an essential receptor for RSV, and drugs targeting its RNA-binding domain can effectively inhibit RSV infection [6].
3.4 Deoxyguanosine Nucleotide Synthesis Pathway
Some studies have shown that inhibiting the deoxyguanosine nucleotide synthesis pathway in host cells can also effectively suppress RSV replication. For example, probenecid, an FDA-approved drug, inhibits RSV replication by blocking organic anion transporters (OATs) [7].
4. Recombinant Proteins Related to RSV Research
| Target |
Product Name |
Source |
Tag Info |
Product Code |
| N |
Recombinant Bovine respiratory syncytial virus Nucleoprotein (N) |
E.coli |
N-terminal 6xHis-SUMO-tagged |
CSB-EP339612BKT |
| F |
Recombinant Human respiratory syncytial virus A Fusion glycoprotein F0 (F), partial |
E.coli |
N-terminal 6xHis-B2M-tagged |
CSB-EP356041HPO |
| F |
Recombinant Human respiratory syncytial virus A Fusion glycoprotein F0 (F), partial |
E.coli |
N-terminal 6xHis-tagged |
CSB-EP356041HPOa0 |
| F |
Recombinant Human respiRatory syncytial virus A Fusion glycoprotein F0 (F), partial |
Yeast |
N-terminal 6xHis-tagged |
CSB-YP356041HPO |
| G |
Recombinant Human respiratory syncytial virus A Major surface glycoprotein G (G), partial |
Baculovirus |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
CSB-BP365937HPO |
| G |
Recombinant Human respiratory syncytial virus A Major surface glycoprotein G (G), partial |
E.coli |
N-terminal 6xHis-SUMO-tagged |
CSB-EP365937HPO |
| G |
Recombinant Human respiratory syncytial virus A Major surface glycoprotein G (G), partial, Biotinylated |
E.coli |
N-terminal 6xHis-SUMO3-Avi-tagged |
CSB-EP365937HPOm4-B |
| F |
Recombinant Human respiratory syncytial virus A Fusion glycoprotein F0 (F), partial |
E.coli |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
CSB-EP319265HPWb1 |
| G |
Recombinant Human respiratory syncytial virus B Major surface glycoprotein G (G), partial |
E.coli |
N-terminal 6xHis-tagged |
CSB-EP323293HPX |
| N |
Recombinant Human respiratory syncytial virus B Nucleoprotein (N) |
E.coli |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
CSB-EP340633HPX |
| F |
Recombinant Human respiRatory syncytial virus B Fusion glycoprotein F0 (F), partial |
Yeast |
N-terminal 6xHis-tagged |
CSB-YP516611HXK |
| M2-1 |
Recombinant Human respiratory syncytial virus B Matrix M2-1 (M2-1) |
E.coli |
N-terminal 6xHis-tagged and C-terminal 6xHis-tagged |
CSB-EP518618HXK |
References:
[1] Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science, 2009.
[2] Oral GS-5806 Activity in a Respiratory Syncytial Virus Challenge Study. The New England Journal of Medicine, 2014.
[3] A Randomized, Placebo-Controlled, Respiratory Syncytial Virus Human Challenge Study of the Antiviral Efficacy, Safety, and Pharmacokinetics of RV521, an Inhibitor of the RSV-F Protein. Antimicrobial Agents and Chemotherapy, 2020.
[4] Structure-Based Discovery of Allosteric Inhibitors Targeting a New Druggable Site in the Respiratory Syncytial Virus Polymerase. ACS Omega, 2024.
[5] Progress in studies on drugs and their target points against respiratory syncytial virus. Chinese Journal of Clinical Hepatology, 2020.
[6] Identification of RSV Fusion Protein Interaction Domains on the Virus Receptor, Nucleolin. Viruses, 2021.
[7] Probenecid Inhibits Respiratory Syncytial Virus (RSV) Replication. Viruses, 2022.

