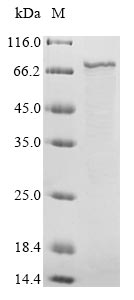
In the production of recombinant hRSV F protein, the gene for F (E.coli) was cloned into a vector and expressed as F protein in E.coli. The plasmids with the copy of F, or the expression vector, were often used to enhance gene expression. Every step of production was undergone with a strict QC system. N-terminal 6xHis-B2M tag was used in the process. The purity is 90% determined by SDS-PAGE.
F is synthesized as a precursor (F0) that must be proteolytically cleaved at polybasic residues, to generate the biologically active forms (F1 and F2). The F1 polypeptide exposes a fusion peptide, whose , whose function is to be inserted into target membrane. HN is thought to be implicated in the activation of F, possibly through direct interactions. The F2 subunit region was also demonstrated to play an important role in the activation of F. Although the fusion protein was found on the infected cell surface, it did not appear to be proteolytically cleaved to F1 and F2 subunits. Immunization of hamsters with the recombinant protein elicited antibody which neutralized infectivity and blocked fusion of virus-infected cells. Human parainfluenza viruses (hPIV) are pathogens responsible for upper and lower respiratory tract infections. Clinical variant strains of hPIV-2 that display unusual large syncytial cytopathic effects. Studies found that F (A96T) mutation strongly alters fusogenic properties of F hPIV-2, highlighting this key residue in the F2 subunit and its possible role in fusion regulation.