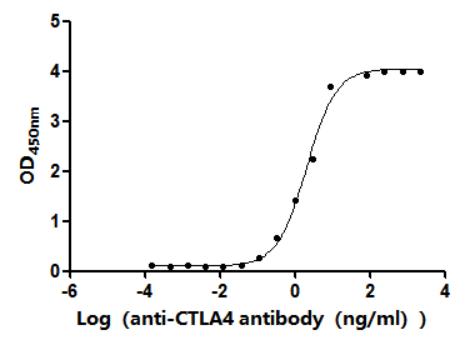
The recombinant Finegoldia magna ATCC 53516 LPXTG-motif cell wall anchor domain protein is co-expressed with the C-terminal 6xHis-tag in E. coli. Its expression region encompasses the amino acid sequence from 106 to 470 of Finegoldia magna ATCC 53516 LPXTG-motif cell wall anchor domain protein. This protein exhibits a purity level exceeding 85% as confirmed by SDS-PAGE analysis. It is provided in a lyophilized powder form, which facilitates storage and handling. Functional assays have demonstrated that this LPXTG-motif cell wall anchor domain protein can effectively bind to anti-protein L antibody (CSB-RA006163MA1HU), with the EC50 ranging from 42.28 to 65.76 ng/mL, indicating its strong affinity for the antibody.
The LPXTG-motif cell wall anchor domain present in Finegoldia magna ATCC 53516 plays crucial roles in the stability and functionality of surface proteins important for the bacterium's adherence and interaction within its ecological niche. As a Gram-positive anaerobic coccus, F. magna utilizes this motif to anchor proteins covalently to the peptidoglycan layer of its cell wall, a process catalyzed by sortase enzymes. The LPXTG motif, recognized by sortase, facilitates the attachment of surface proteins critical for bacterial adherence and pathogenicity [1].
This anchoring mechanism is particularly significant for proteins involved in host interactions. Surface proteins carrying the LPXTG motif can modulate the host immune response, and some studies indicate that these proteins can act as virulence factors. For example, F. magna expresses superantigen Protein L, which can induce inflammation by activating human neutrophils and promoting an inflammatory response [2][3]. Moreover, the LPXTG domain also contributes to the diverse functionality of surface proteins that can interact with host cells, facilitating adhesion and colonization within the gastrointestinal tract [1][3].
Additionally, the surface localization of LPXTG-anchored proteins has been linked to bacterial growth regulation, suggesting that such motifs are instrumental not only for anchorage but also for the dynamic regulation of protein localization during the bacterial cell cycle [4]. The variation in the localization patterns of these proteins during cell division further points to their significant role in maintaining cellular integrity and contributing to the overall pathogenicity of Gram-positive bacteria like F. magna [4].
In summary, the LPXTG-motif cell wall anchor domain in Finegoldia magna is integral in linking various surface proteins to the cell wall, which supports bacterial adherence, modulates host immune responses, and is essential for maintaining cellular functions relevant to growth and virulence.
References:
[1] T. Goto, A. Yamashita, et al. Complete genome sequence of finegoldia magna, an anaerobic opportunistic pathogen. Dna Research, vol. 15, no. 1, p. 39-47, 2008. https://doi.org/10.1093/dnares/dsm030
[2] A. Neumann, L. Björck, & I. Frick. Finegoldia magna, an anaerobic gram-positive bacterium of the normal human microbiota, induces inflammation by activating neutrophils. Frontiers in Microbiology, vol. 11, 2020. https://doi.org/10.3389/fmicb.2020.00065
[3] M. Zou, Z. Yang, et al. Gut microbiota on admission as predictive biomarker for acute necrotizing pancreatitis. Frontiers in Immunology, vol. 13, 2022. https://doi.org/10.3389/fimmu.2022.988326
[4] G. Mathiesen, L. Øverland, K. Kuczkowska, & V. Eijsink. Anchoring of heterologous proteins in multiple lactobacillus species using anchors derived from lactobacillus plantarum. Scientific Reports, vol. 10, no. 1, 2020. https://doi.org/10.1038/s41598-020-66531-7