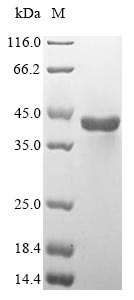
To make this Recombinant Human ASPA protein, the ASPA gene was isolated at first and cloned into an expression vector. CUSABIO has built a mature recombinant protein platform. This Recombinant Human ASPA protein was developed in the platform. It was expressed in Mammalian cell at the region of 1-313aa of the Human ASPA protein. N-terminal 10xHis tag and C-terminal Myc tag was fused with the expression vector for affinity and purification purposes. The purity is 85%+ determined by SDS-PAGE.
ASPA is an enzyme reported to be involved in the hydrolysis of N-acetyl-aspartate (NAA) into acetate and aspartate. A reduction in free acetate for lipid synthesis subsequent to loss of ASPA function is believed to contribute to disease etiology and could likely account for abnormalities in the lipid content of myelin. Early observations suggested an association of Canavan disease with oligodendrocytes due to loss of white matter as a result of the absence of functional ASPA. Thus, modification of aspartoacylase might be serve as a potential use in enzyme replacement therapy for the treatment of Canavan disease. Besides, extensive aspartoacylase expression in the rat central nervous system. Several findings provide strong support for a carboxypeptidase-type mechanism for the hydrolysis of the amide bond of the substrate, N-acetyl- l-aspartate.