Enhance your signal transduction research with our high-quality Recombinant Human ASPA protein. Aspartoacylase, also known as Aminoacylase-2 or ACY-2, is a crucial enzyme responsible for the hydrolysis of N-acetyl-L-aspartic acid (NAA) into aspartate and acetate within the central nervous system. This enzyme has significant implications in neural development, myelin maintenance, and various neurodegenerative disorders.
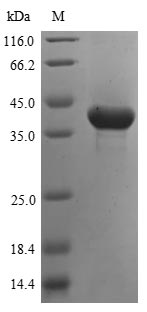
Derived from yeast expression systems, our recombinant ASPA protein is a full-length construct spanning the 1-313aa region, specifically designed to facilitate your scientific investigations. The N-terminal 10xHis-tag and C-terminal Myc-tag enable seamless protein purification and detection. With a purity greater than 85% as determined by SDS-PAGE, our Recombinant Human ASPA protein is provided in a lyophilized powder format, ensuring optimal results in your signal transduction studies.