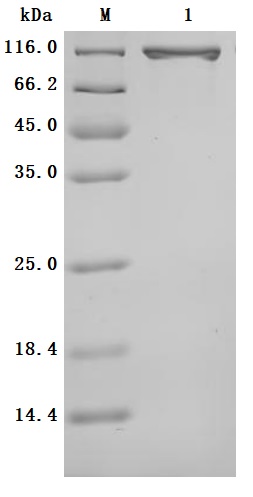
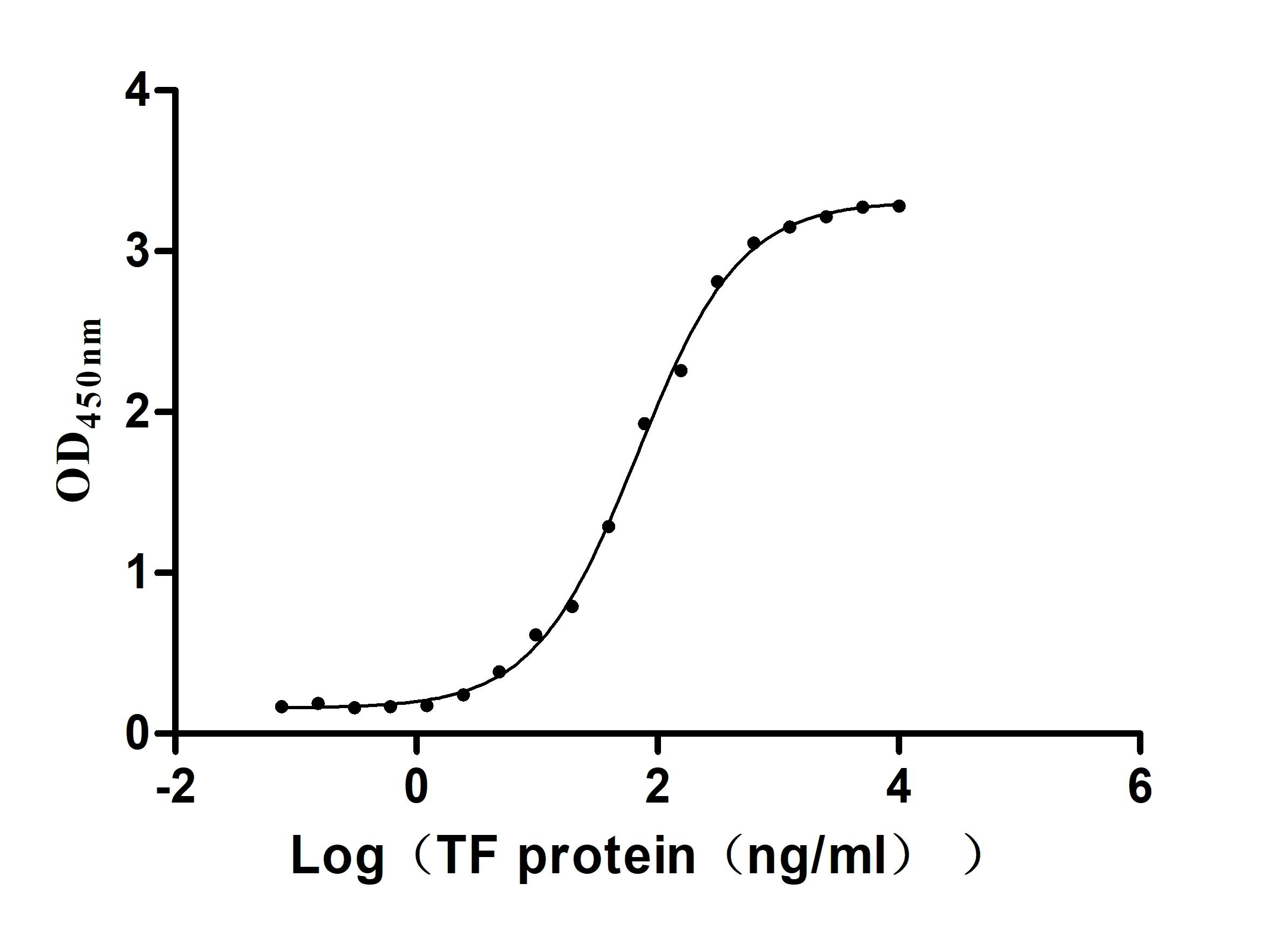
The production pipeline for the recombinant human serotransferrin (TF) includes gene cloning, plasmid construction, protein expression, and quality control. It is expressed in the mammalian cells. Its expression region corresponds to the 20-698aa of the human serotransferrin. It also carries a C-terminal hFc-tag. It is subjected to affinity chromatography purification. Its purity is over 95% as measured by SDS-PAGE, and its endotoxin levels are less than 1.0 EU/μg as accessed by the LAL method. This recombinant serotransferrin is validated to be biologically active. In a functional ELISA, the human TFRC (CSB-MP3648HU) can bind to this serotransferrin, with an EC50 of 58.72-77.84 ng/mL.
Human serotransferrin, also known as serum transferrin, is a glycoprotein primarily synthesized in the liver, playing a crucial role in iron transport and homeostasis. Serotransferrin is responsible for binding iron ions and facilitating their delivery to various tissues through receptor-mediated endocytosis, which is essential for cellular functions and overall iron homeostasis in the body [1][2].
The structure of human serotransferrin is characterized by its ability to bind two iron ions per molecule, which is vital for its function in iron transport. Serotransferrin is composed of two homologous lobes, each capable of binding an iron ion [2][3]. The glycosylation patterns of serotransferrin are also significant, as they can influence its biological activity and interactions with other proteins. For instance, variations in glycan composition have been linked to different pathological conditions, including liver diseases and cancers [4].
Elevated levels of serotransferrin have been associated with certain cancers, such as breast cancer and hepatocellular carcinoma [5][6]. Additionally, serotransferrin is involved in the acute-phase response, where its concentration can increase in response to inflammation or tissue injury, further emphasizing its importance in disease states [7]. Research has also shown that serotransferrin levels are altered in various conditions, including glaucoma and chronic kidney disease, suggesting serotransferrin's role in the pathogenesis of these diseases [7][8]. Furthermore, its role in iron metabolism makes it a critical factor in understanding diseases related to iron deficiency or overload, such as hemochromatosis and anemia [1].
References:
[1] H. Mohd-Padil, A. Mohd-Adnan, & T. Gabaldón. Phylogenetic analyses uncover a novel clade of transferrin in nonmammalian vertebrates, Molecular Biology and Evolution, vol. 30, no. 4, p. 894-905, 2012. https://doi.org/10.1093/molbev/mss325
[2] A. Hughes and R. Friedman. Evolutionary diversification of the vertebrate transferrin multi-gene family, Immunogenetics, vol. 66, no. 11, p. 651-661, 2014. https://doi.org/10.1007/s00251-014-0798-x
[3] G. Lee. Dual differential roles of cancerous immunoglobulins as suggested by interactions with human serum proteins, Journal of Clinical & Cellular Immunology, vol. 06, no. 06, 2015. https://doi.org/10.4172/2155-9899.1000385
[4] W. Jamnongkan, C. Lebrilla, M. Barboza, A. Techasen, W. Loilome, P. Sithithaworn, et al. Discovery of serotransferrin glycoforms: novel markers for diagnosis of liver periductal fibrosis and prediction of cholangiocarcinoma, Biomolecules, vol. 9, no. 10, p. 538, 2019. https://doi.org/10.3390/biom9100538
[5] S. Selvaraju and Z. Rassi. Targeting human serum fucome by an integrated liquid‐phase multicolumn platform operating in “cascade” to facilitate comparative mass spectrometric analysis of disease‐free and breast cancer sera, Proteomics, vol. 13, no. 10-11, p. 1701-1713, 2013. https://doi.org/10.1002/pmic.201200524
[6] M. Rantalainen, O. Cloarec, O. Beckonert, I. Wilson, D. Jackson, R. Tonge, et al. Statistically integrated metabonomic−proteomic studies on a human prostate cancer xenograft model in mice, Journal of Proteome Research, vol. 5, no. 10, p. 2642-2655, 2006. https://doi.org/10.1021/pr060124w
[7] S. Reinehr, A. Guntermann, J. Theile, L. Benning, P. Grotegut, S. Kuehn, et al. Proteomic analysis of retinal tissue in an s100b autoimmune glaucoma model, Biology, vol. 11, no. 1, p. 16, 2021. https://doi.org/10.3390/biology11010016
[8] A. Taherkhani, R. Yekta, M. Mohseni, M. Saidijam, & A. Oskouie. Chronic kidney disease: a review of proteomic and metabolomic approaches to membranous glomerulonephritis, focal segmental glomerulosclerosis, and iga nephropathy biomarkers, Proteome Science, vol. 17, no. 1, 2019. https://doi.org/10.1186/s12953-019-0155-y