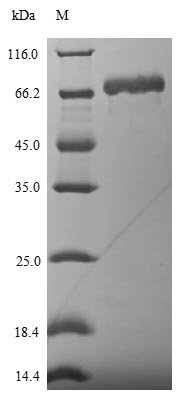
Recombinant Influenza A virus Nucleoprotein (NP) is expressed in E. coli and spans the full length of the protein, covering amino acids 1 to 498. The protein carries an N-terminal 6xHis-SUMO tag to aid purification and improve solubility. SDS-PAGE analysis confirms a purity level greater than 90%, which appears suitable for various research applications. This product is designated for research use only.
The nucleoprotein (NP) of the Influenza A virus represents a critical component in the viral replication cycle. It wraps around the viral RNA genome and plays a vital role in assembling ribonucleoprotein complexes. This protein is essential for transcription, replication, and packaging of viral RNA—making it a key target for influenza research and antiviral strategies. Understanding NP functions may help scientists study viral pathogenesis and immune responses.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Influenza A virus nucleoprotein (NP) is a viral protein that forms oligomers and binds RNA as part of the viral ribonucleoprotein complex. The E. coli expression system can often produce soluble viral proteins, and the SUMO tag may enhance solubility and folding. However, NP requires specific oligomerization and RNA-binding capabilities for full functionality. The large N-terminal SUMO tag (∼15 kDa) may sterically interfere with proper oligomerization or RNA-binding interfaces. While the protein may be soluble and partially folded, the probability of correct oligomerization and functional RNA-binding activity is moderate but requires experimental validation.
1. Antibody Development and Characterization
This recombinant NP serves as an excellent immunogen for generating antibodies against influenza A nucleoprotein. The full-length sequence ensures comprehensive coverage of the epitope. The SUMO tag facilitates purification and immunization procedures. These antibodies will be valuable for detecting NP in viral research applications.
2. Protein-Protein Interaction Studies
Protein-protein interactions in the viral replication complex require a precise quaternary structure that may be compromised by the large tag. The SUMO tag may sterically hinder interaction interfaces with viral proteins (like PB1, PB2, PA) or host factors. A misfolded NP may exhibit non-specific binding. If correctly oligomerized, it could identify physiological partners, but results require validation with complementary methods. Any interaction data would be biologically irrelevant without validation using a natively folded protein.
3. ELISA-Based Binding Assays
This protein is highly suitable as a coating antigen for ELISA to detect anti-NP antibodies. The His-tag enables consistent immobilization on nickel-coated plates. However, functional binding assays (e.g., with viral polymerase proteins) would require proper folding and oligomerization, which are unknown and need to be verified.
4. Biochemical Characterization Studies
This is the essential first step to assess protein quality. Techniques like size-exclusion chromatography with multi-angle light scattering (SEC-MALS) can determine oligomeric state, while RNA-binding assays can validate functionality. SUMO protease cleavage allows study of tag-free protein properties. These analyses provide crucial data on folding state, oligomerization, and functional potential of this protein itself, not the native protein.
Final Recommendation & Action Plan
This recombinant NP has strong potential for immunological applications but requires validation of oligomerization and RNA-binding activity before reliable use in interaction studies. The immediate priority is Application 4 (Biochemical Characterization) to assess oligomeric state via SEC-MALS and validate RNA-binding capability. If proper oligomerization is confirmed, proceed cautiously with Application 2 (Interaction Studies). Applications 1 and 3 (Antibody Development and ELISA) can proceed immediately. Consider SUMO tag removal for critical functional studies. For reliable viral replication complex studies, validation with native NP from viral particles is recommended. This systematic approach ensures appropriate use based on functional validation.