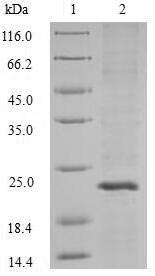
Recombinant Streptococcus pyogenes serotype M1 Adenine phosphoribosyltransferase is produced using the baculovirus expression system. This full-length protein spans amino acids 1 to 172 and comes tagged with an N-terminal 10xHis-tag and a C-terminal Myc-tag, which appears to make purification and detection more straightforward. SDS-PAGE analysis indicates a purity greater than 90%, suggesting it may be suitable for various research applications.
Adenine phosphoribosyltransferase is an enzyme that works within the purine salvage pathway, where it catalyzes the conversion of adenine to AMP. This protein likely plays an important role in nucleotide metabolism by helping recycle adenine efficiently. Studying it could shed light on purine metabolism and might offer insights into bacterial physiology—perhaps even revealing potential antimicrobial targets.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Antibody Development and Immunoassay Studies
This recombinant protease serves as an excellent immunogen for generating specific antibodies. The full-length sequence ensures comprehensive epitope coverage. Even if the protease is misfolded or inactive, it will still generate antibodies against linear epitopes useful for techniques like Western blotting. The high purity and dual tags facilitate efficient immunization and screening.
2. Protein-Protein Interaction Studies
The utility for interaction studies is highly dependent on the correct folding of this APT protein. A properly folded protease could identify physiological substrates or regulators. However, a misfolded protease may expose non-specific hydrophobic surfaces, leading to false-positive interactions, or mask authentic binding sites, causing false-negatives. Data would be uninterpretable without folding validation. Protease-substrate/inhibitor interactions require a precise active site conformation. Using a protease of unknown folding state carries an unacceptably high risk of generating artifactual, misleading results.
3. Enzymatic Activity Assays and Inhibitor Screening
This application is entirely contingent on the protease being both correctly folded and active. If functional, this recombinant APT protein would be vital for characterizing kinetics, substrate specificity, and screening inhibitors. However, without activity confirmation, any assay will yield meaningless results, wasting resources and potentially leading to incorrect conclusions.
4. Biochemical Characterization and Folding Analysis
This application is the essential first step to determine the protein's folding state and stability. Techniques like size-exclusion chromatography (SEC) can assess oligomeric state and homogeneity, while circular dichroism (CD) can analyze secondary structure. These studies provide critical data on whether the protein is natively folded—a prerequisite for any functional study.
Final Recommendation & Action Plan
The unknown folding state and enzymatic activity mandate a strict, sequential approach. The immediate and mandatory first step is Application 4 (Biochemical Characterization) to assess folding quality via SEC and CD. If the protein is monodisperse and has a structured spectrum, the next critical step is to perform a basic enzymatic activity assay using a fluorogenic or chromogenic substrate to confirm functionality. Only after successfully confirming both correct folding and enzymatic activity can the protein be responsibly used for Application 3 (Enzymatic Assays) and Application 2 (Interaction Studies). Until this validation is complete, Application 1 (Antibody Development) is the only safe and reliable use for this protein reagent. Investing in functional studies before this validation is high-risk and likely to generate unreliable data.