Breast Cancer
Breast cancer, a malignant tumor originating in the epithelial cells of the breast, is one of the most common cancers among women and one of the leading causes of cancer deaths in women. The incidence of breast cancer is related to a number of factors, including heredity, age, hormone levels, and lifestyle. Based on the characteristics of tumor cells, breast cancer can be classified into different subtypes, such as hormone receptor positive (ER+/PR+), human epidermal growth factor receptor 2 (HER2+), and triple-negative breast cancer (TNBC).
In recent years, significant progress has been made in breast cancer treatment and drug development. Especially in the field of targeted therapy and immunotherapy, the continuous emergence of new drugs and therapies has brought more treatment options and better prognosis for patients.
Targeted therapy drugs:
● HER2 positive breast cancer
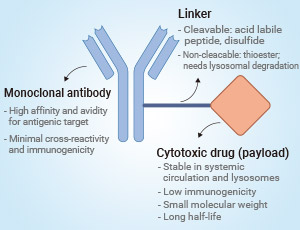
The treatment of HER2-positive breast cancer has made significant progress in the past few years.ADC (Antibody Drug Coupling Compound)-based drug therapy is an important breakthrough in the treatment of HER2-positive breast cancer. For example, DS-8201 (Trastuzumab Deruxtecan), an ADC drug targeting HER2, has shown potential efficacy in clinical trials in patients with low HER2 expression and HER2-negative breast cancer.67 Additionally, T-DM1 (Trastuzumab Emtansine) and T-DXd ( Trastuzumab Deruxtecan) have also been successful in the treatment of HER2-positive breast cancer.
● Hormone receptor positive breast cancer
For hormone receptor-positive breast cancer, the development of CDK4/6 inhibitors such as abemaciclib, dalciclib, and ribociclib has provided new treatment options for patients. These drugs slow the growth and spread of tumor cells by inhibiting key regulators of the cell cycle.
● Triple Negative Breast Cancer
Triple-negative breast cancer (TNBC) is one of the more difficult subtypes to treat because it lacks hormone receptors and HER2 expression. However, immunotherapy has made progress in this area, for example PD-1/PD-L1 inhibitors have shown potential in the treatment of TNBC.
Immunotherapy drugs:
Immunotherapy is another important advance in the field of breast cancer treatment. Immune checkpoint inhibitors, such as PD-1 and PD-L1 inhibitors, have shown some efficacy in the treatment of triple negative breast cancer. In addition, researchers are exploring strategies to use immunotherapy in conjunction with other therapies to improve treatment outcomes.
Breast Cancer Drug Targets
Breast Cancer Drug Target Related Products
● Target Proteins
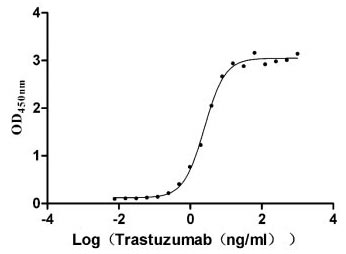
Measured by its binding ability in a functional ELISA. Immobilized HER2 at 2 μg/ml can bind Trastuzumab, the EC50 is 2.179-2.825 ng/ml.
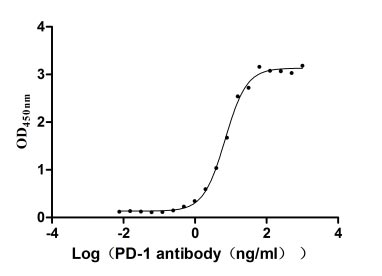
Measured by its binding ability in a functional ELISA. Immobilized PD-1 at 2 μg/ml can bind Anti-PD-1 recombinant antibody, the EC50 of human PD-1 protein is 6.087-7.854 ng/ml.
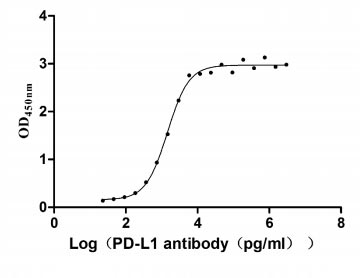
Measured by its binding ability in a functional ELISA. Immobilized PD-L1 at 2 μg/ml can bind Anti- PD-L1 mouse monoclonal antibody (CSB-MA878942A1m, antigen from E.coli), the EC50 of human PD-L1 protein is 1.252-1.653 ng/mL
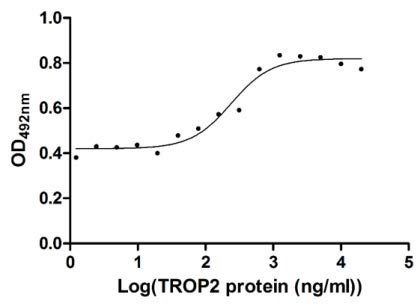
Measured in cell activity assay using U937 cells, the EC50 for this effect is 190.2-298.6 ng/ml.
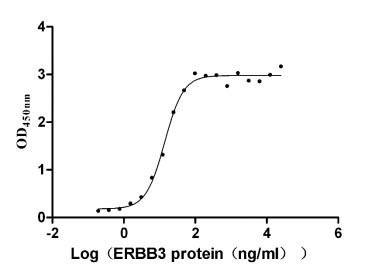
Measured by its binding ability in a functional ELISA. Immobilized NRG1 (CSB-MP016077HU1(F6)) at 2 μg/ml can bind human ERBB3, the EC50 is 12.32-15.74 ng/ml.
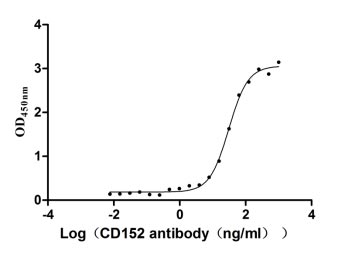
Measured by its binding ability in a functional ELISA. Immobilized CD152 at 2 μg/ml can bind Anti-CD152 rabbit monoclonal antibody (CSB-RA213310A0HU), the EC50 of human CD152 protein is 27.14-34.82 ng/ml.
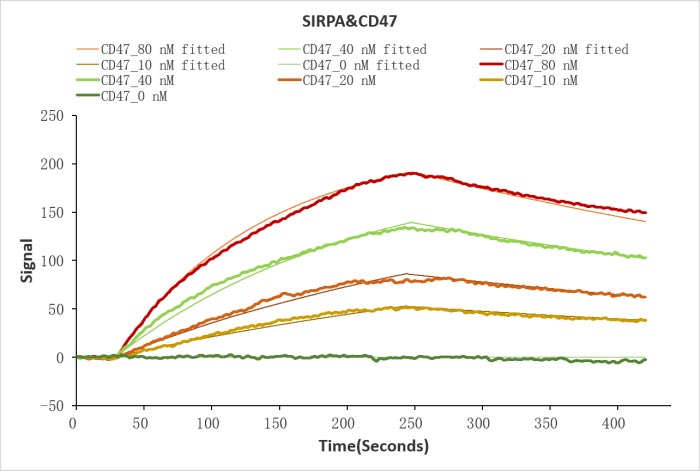
Human SIRPA protein His/Myc tag (CSB-MP021334HU) captured on COOH chip can bind Human CD47 protein Fc tag (CSB-MP004940HU) with an affinity constant of 19.1 nM as detected by LSPR Assay.
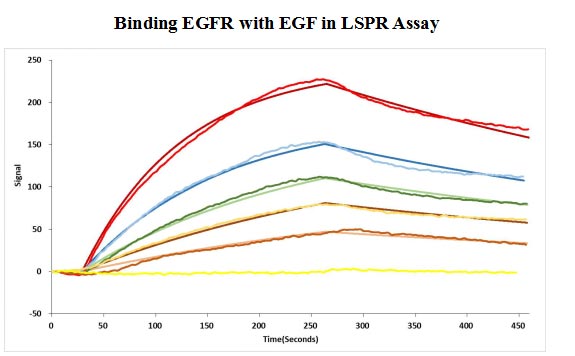
Human EGF protein captured on COOH chip can bind Human EGFR protein, his and Myc tag (CSB-MP007479HU) with an affinity constant of 11.9nM as detected by LSPR Assay.
| Product Name |
Code |
Target |
Source |
Tag Info |
| Recombinant Human Programmed cell death 1 ligand 1 (CD274), partial (Active) |
CSB-MP878942HU1 |
CD274 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human CD276 antigen (CD276), partial (Active) |
CSB-MP733578HU |
CD276 |
Mammalian cell |
C-terminal hFc-Myc-tagged |
| Recombinant Human Tumor necrosis factor receptor superfamily member 5 (CD40), partial (Active) |
CSB-MP004936HU1 |
CD40 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Macaca fascicularis CD44 antigen (CD44), partial (Active) |
CSB-MP4290MOV |
CD44 |
Mammalian cell |
C-terminal 10xHis-tagged |
| Recombinant Human CD44 antigen (CD44), partial, Biotinylated (Active) |
CSB-MP004938HU(F1)j1-B |
CD44 |
Mammalian cell |
C-terminal mFc-Avi-tagged |
| Recombinant Human CD44 antigen (CD44), partial (Active) |
CSB-MP004938HU(F1) |
CD44 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human Leukocyte surface antigen CD47 (CD47), partial (Active) |
CSB-MP004940HU |
CD47 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) (E398K) (Active) |
CSB-MP005165HU |
CEACAM5 |
Mammalian cell |
C-terminal 10xHis-tagged |
| Recombinant Human Cytotoxic T-lymphocyte protein 4 (CTLA4), partial (Active) |
CSB-MP006163HU1 |
CTLA4 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human Epidermal growth factor receptor (EGFR), partial (Active) |
CSB-MP007479HU |
EGFR |
Mammalian cell |
N-terminal 10xHis-tagged and C-terminal Myc-tagged |
| Recombinant Human Receptor tyrosine-protein kinase erbB-2 (ERBB2), partial (Active) |
CSB-MP007763HU |
ERBB2 |
Mammalian cell |
C-terminal 6xHis-tagged |
| Recombinant Human Receptor tyrosine-protein kinase erbB-3 (ERBB3), partial (Active) |
CSB-MP007765HU |
ERBB3 |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human Interleukin-2 (IL2) (Active) |
CSB-MP011629HU |
IL2 |
Mammalian cell |
N-terminal hFc-tagged |
| Recombinant Human Interleukin-2 receptor subunit alpha (IL2RA), partial (Active) |
CSB-MP011649HU3 |
IL2RA |
Mammalian cell |
C-terminal 10xHis-tagged |
| Recombinant Human Hepatocyte growth factor receptor (MET), partial (Active) |
CSB-MP013714HU |
MET |
Mammalian cell |
C-terminal hFc-tagged |
| Recombinant Human Mesothelin (MSLN), partial (Active) |
CSB-MP015044HUc9 |
MSLN |
Mammalian cell |
N-terminal hFc-tagged |
| Recombinant Human Mucin-16 (MUC16), partial (Active) |
CSB-MP704410HU3c7 |
MUC16 |
Mammalian cell |
C-terminal 6xHis-tagged |
| Recombinant Human Nectin-4 (NECTIN4), partial (Active) |
CSB-MP822274HU |
NECTIN4 |
Mammalian cell |
C-terminal 10xHis-tagged |
| Recombinant Human 5'-nucleotidase (NT5E) (Active) |
CSB-MP723415HU |
NT5E |
Mammalian cell |
C-terminal 6xHis-tagged |
| Recombinant Human Programmed cell death protein 1 (PDCD1), partial (Active) |
CSB-MP619964HU1 |
PDCD1 |
Mammalian cell |
C-terminal 6xHis-tagged |
● Cell Lines
● Recombinant antibodies
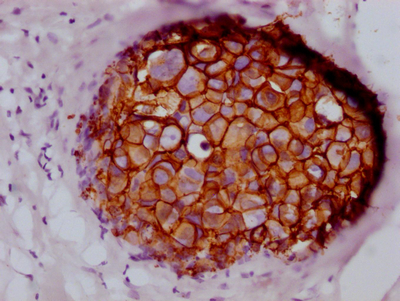
IHC image of CSB-RA260392A0HU diluted at 1:100 and staining in paraffin-embedded human breast cancer performed on a Leica BondTM system.
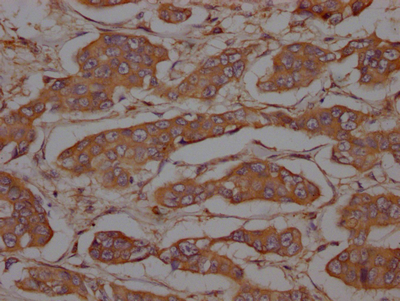
IHC image of CSB-RA634199A0HU diluted at 1:100 and staining in paraffin-embedded human breast cancer performed on a Leica BondTM system.
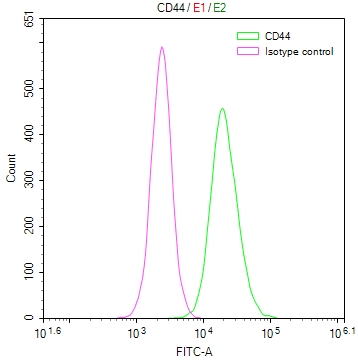
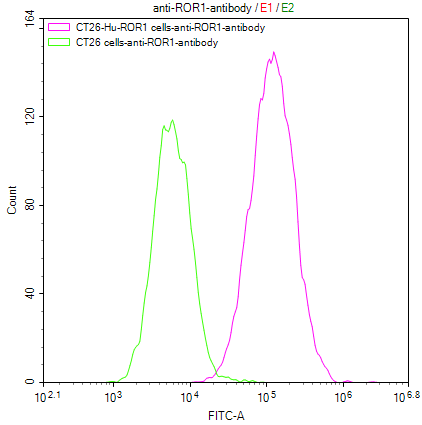
Overlay Peak curve showing Hela cells surface stained with CSB-RA292372A0HU (red line) at 1:50.
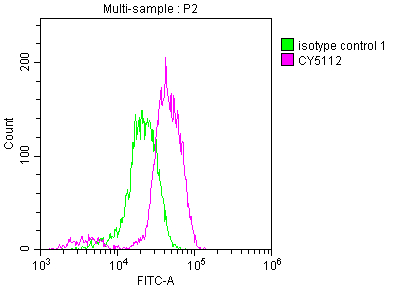
Overlay histogram showing Jurkat cells stained with CSB-RA159341A0HU (red line) at 1:50.
| Product Name |
Code |
Target |
Species Reactivity |
Tested Applications |
| CA9 Recombinant Monoclonal Antibody |
CSB-RA614990A0HU |
CA9 |
Human |
ELISA, IHC |
| CD247 Recombinant Monoclonal Antibody |
CSB-RA244537A0HU |
CD247 |
Human |
ELISA, FC |
| CD27 Recombinant Monoclonal Antibody |
CSB-RA953976A0HU |
CD27 |
Human |
ELISA, WB |
| CD274 Recombinant Monoclonal Antibody |
CSB-RA977797A0HU |
CD274 |
Human |
ELISA, WB, IHC |
| CD274 Recombinant Monoclonal Antibody |
CSB-RA878942MA1HU |
CD274 |
Human |
ELISA, IHC, FC |
| CD40 Recombinant Monoclonal Antibody |
CSB-RA004936MA1HU |
CD40 |
Human |
ELISA, IF, FC |
| CD44 Recombinant Monoclonal Antibody |
CSB-RA004938A0HU |
CD44 |
Human |
ELISA, WB, IHC |
| CD44 Recombinant Monoclonal Antibody |
CSB-RA292372A0HU |
CD44 |
Human |
ELISA, WB, IHC, IF, FC |
| CD44 Recombinant Monoclonal Antibody |
CSB-RA004938MA1HU |
CD44 |
Human |
ELISA, FC |
| CD47 Recombinant Monoclonal Antibody |
CSB-RA802124A0HU |
CD47 |
Human |
ELISA, WB, IHC, IF |
| CEACAM5 Recombinant Monoclonal Antibody |
CSB-RA005165MA3HU |
CEACAM5 |
Human |
ELISA, IF, FC |
| CEACAM5 Recombinant Monoclonal Antibody |
CSB-RA005165MA1HU |
CEACAM5 |
Human |
ELISA |
| CTLA4 Recombinant Monoclonal Antibody |
CSB-RA213310A0HU |
CTLA4 |
Human |
ELISA, IHC |
| CTLA4 Recombinant Monoclonal Antibody |
CSB-RA006163MA1HU |
CTLA4 |
Human, Mouse |
ELISA, WB, IF, FC |
| Phospho-EGFR (Y1092) Recombinant Monoclonal Antibody |
CSB-RA007479A1092phHU |
EGFR |
Human |
ELISA, WB |
| Phospho-EGFR (Y1068) Recombinant Monoclonal Antibody |
CSB-RA007479A1068phHU |
EGFR |
Human |
ELISA, WB |
| EGFR Recombinant Monoclonal Antibody |
CSB-RA159341A0HU |
EGFR |
Human |
ELISA, WB, IHC, IF, FC |
| EGFR Recombinant Monoclonal Antibody |
CSB-RA794061A0HU |
EGFR |
Human |
ELISA, WB, IHC |
| EGFR Recombinant Monoclonal Antibody |
CSB-RA159341MA1HU |
EGFR |
Human |
ELISA, IHC, FC |
| EPCAM Recombinant Monoclonal Antibody |
CSB-RA932207A0HU |
EPCAM |
Human |
ELISA, WB |
● ELISA Kits
| Product Name |
Code |
Target |
Detection Range |
Sensitivity |
| Human Carbonic Anhydrase 9(CA9) ELISA Kit |
CSB-E13266h |
CA9 |
31.25 pg/mL-2000 pg/mL |
7.81 pg/mL |
| Human T-cell surface glycoprotein CD3 zeta chain (CD247) ELISA kit |
CSB-EL004904HU |
CD247 |
0.156 ng/mL-10 ng/mL |
0.039 ng/mL |
| Human Programmed Death Ligand-1(PD-L1/CD274) ELISA Kit |
CSB-E13644h |
CD274 |
15.6 pg/mL-1000 pg/mL |
3.9 pg/mL |
| Mouse Programmed cell death 1 ligand 1(CD274) ELISA kit |
CSB-EL004911MO |
CD274 |
31.25 pg/mL-2000 pg/mL |
7.81 pg/mL |
| Human Soluble CD276,sCD276/sB7-H3 ELISA Kit |
CSB-E14285h |
CD276 |
3.12 ng/mL-200 ng/mL |
0.78 ng/mL |
| Human soluble cluster of differentiation 28,sCD28 ELISA Kit |
CSB-E09296h |
CD28 |
0.625 ng/mL-40 ng/mL |
0.156 ng/mL |
| Mouse Tumor necrosis factor receptor superfamily member 5(CD40) ELISA kit |
CSB-EL004936MO |
CD40 |
Request Information |
Request Information |
| Human cluster Of differentiation,CD44 ELISA Kit |
CSB-E11846h |
CD44 |
78 pg/mL-5000 pg/mL |
19.5 pg/mL |
| Human Leukocyte surface antigen CD47(CD47) ELISA kit |
CSB-EL004940HU |
CD47 |
3.12 pg/mL-200 pg/mL |
0.78 pg/mL |
| Mouse Leukocyte surface antigen CD47(CD47) ELISA kit |
CSB-EL004940MO |
CD47 |
15.6 pg/mL-1000 pg/mL |
3.9 pg/mL |
| Human carcinoembryonic antigen,CEA ELISA Kit |
CSB-E04767h |
CEACAM5 |
5 ng/mL-120 ng/mL |
2 ng/mL |
| Mouse carcinoembryonic antign,CEA ELISA Kit |
CSB-E13925m |
CEACAM5 |
62.5 pg/mL-4000 pg/mL |
15.6 pg/mL |
| Human Macrophage Colony-Stimulating Factor Receptor,M-CSFR ELISA Kit |
CSB-E10012h |
CSF1R |
0.156 ng/mL-10 ng/mL |
0.039 ng/mL |
| Rat Macrophage colony-stimulating factor 1 receptor(CSF1R) ELISA kit |
CSB-EL006044RA |
CSF1R |
0.312 ng/mL-20 ng/mL |
0.078 ng/mL |
| Human Granulocyte-Macrophage Colony Stimulating Factor,GM-CSF ELISA Kit |
CSB-E04568h |
CSF2 |
15.6 pg/mL-1000 pg/mL |
3.9 pg/mL |
| Mouse Granulocyte-Macrophage Colony Stimulating Factor,GM-CSF ELISA Kit |
CSB-E04569m |
CSF2 |
15.6 pg/mL-1000 pg/mL |
3.9 pg/mL |
| Rat Granulocyte-Macrophage Colony Stimulating Factor,GM-CSF ELISA Kit |
CSB-E04570r |
CSF2 |
15.6 pg/ml - 1000 pg/ml |
3.9 pg/ml |
| Human cytotoxic T lymphocyte associated antigen 4,CTLA-4 ELISA Kit |
CSB-E09171h |
CTLA4 |
125 pg/mL-8000 pg/mL |
31.25 pg/mL |
| Mouse cytotoxic T lymphocyte associated antigen 4,CTLA-4 ELISA Kit |
CSB-E13637m |
CTLA4 |
0.312 ng/mL-20 ng/mL |
0.078 ng/mL |
| Human epidermal growth factor receptor,EGFR ELISA Kit |
CSB-E12124h |
EGFR |
0.312 ng/mL-20 ng/mL |
0.019 ng/mL |
Drug Development Solutions
References
1. Cell 186, April 13, 2023
2. Larissa A. Korde, Mark R. Somerfield, Dawn L. Hershman, et al. Use of Immune Checkpoint Inhibitor Pembrolizumab in the Treatment of High-Risk, Early-Stage Triple-Negative Breast Cancer: ASCO Guideline Rapid Recommendation Update. DOI: 10.1200/JCO.22.00503 Journal of Clinical Oncology
3. Lajos Pusztai, Carsten Denkert, Joyce O'Shaughnessy, et al. Event-free survival by residual cancer burden after neoadjuvant pembrolizumab + chemotherapy versus placebo + chemotherapy for early TNBC: Exploratory analysis from KEYNOTE-522. J Clin Oncol 40, 2022 (suppl 16; abstr 503). DOI: 10.1200/JCO.2022.40.16_suppl.503
4. Breast cancer-WHO: https://www.who.int/news-room/fact-sheets/detail/breast-cancer