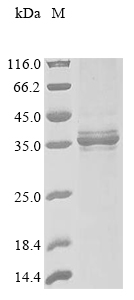
Recombinant Dog Erythropoietin receptor (EPOR) is expressed in E. coli with a partial sequence spanning amino acids 25 to 250. This protein features both N-terminal 6xHis-GB1 and C-terminal 6xHis tags, which aid in purification and detection. The purity level exceeds 85% as verified by SDS-PAGE, ensuring reliable experimental results. This product is designed for research use only and is not suitable for therapeutic or diagnostic applications.
The erythropoietin receptor (EPOR) appears to be a critical component in regulating erythropoiesis—the process of red blood cell production. It works by binding to erythropoietin, triggering a cascade of intracellular signaling pathways that promote cell survival, proliferation, and differentiation. EPOR plays a significant role in studies related to blood cell formation and may offer insight into conditions affecting red blood cell levels.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Dog EPOR is a transmembrane glycoprotein that requires precise folding, proper disulfide bond formation, glycosylation, and specific tertiary structure for its functional activity in ligand binding and signal transduction. The E. coli expression system cannot provide the eukaryotic folding environment, glycosylation machinery, or optimal conditions for correct disulfide bond formation. The dual tags (N-terminal 6xHis-GB1 and C-terminal 6xHis) are large relative to the protein fragment and may cause significant steric interference with the protein's functional domains, particularly the ligand-binding site. The partial fragment (25-250aa) represents only the extracellular domain and lacks critical transmembrane and cytoplasmic regions essential for full receptor functionality. The probability of correct folding with functional bioactivity is extremely low without experimental validation.
1. Antibody Development and Screening
This application is highly suitable as antibody development relies on antigenic sequence recognition rather than functional protein folding. The extracellular domain fragment provides defined epitopes for generating EPOR-specific antibodies. The high purity (>85%) ensures minimal contamination issues. However, antibodies may primarily target linear epitopes and may not recognize conformational epitopes of the native, glycosylated EPOR in its physiological context.
2. Structural and Biophysical Characterization
Basic biophysical analysis can be performed, but will not reflect native EPOR structure. Techniques like circular dichroism spectroscopy or dynamic light scattering will primarily characterize the tag-dominated construct rather than the receptor's extracellular domain. The GB1 tag may enhance solubility but will interfere with native conformational analysis. Results describe an artificial protein, not physiological EPOR.
Final Recommendation & Action Plan
This E. coli-expressed EPOR fragment with large dual tags is fundamentally unsuitable for functional studies due to the lack of eukaryotic folding, glycosylation, and severe tag interference. Antibody development can proceed immediately, but with awareness that antibodies may not recognize native EPOR. Application 2 provides only a basic characterization of the recombinant construct. For reliable EPOR research, use a full-length protein expressed in mammalian systems that supports proper folding, glycosylation, and preserves native conformational epitopes.