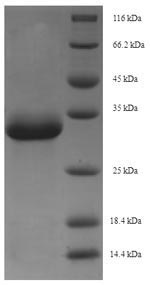
This Recombinant Macaca fascicularis Erythropoietin (EPO) is expressed in E. coli and covers the full length of the mature protein from amino acids 28 to 192. The protein comes with an N-terminal 6xHis-SUMO tag, which makes purification and detection more straightforward. SDS-PAGE analysis shows the product maintains purity levels above 90%, suggesting it's well-suited for most research applications.
Erythropoietin appears to be one of the more important glycoprotein hormones when it comes to controlling red blood cell production. The protein seems to drive erythropoiesis by encouraging both differentiation and growth of erythroid progenitor cells. Researchers have shown considerable interest in EPO's function and related pathways, especially those studying blood formation processes and exploring potential treatments for anemia and similar blood disorders.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
EPO is a complex glycoprotein hormone that requires specific glycosylation patterns for proper folding, stability, and biological activity. The E. coli expression system cannot perform the necessary post-translational modifications (particularly N-linked glycosylation) that are essential for EPO's structural integrity and function. While the SUMO tag may improve solubility, the protein will lack the carbohydrate modifications critical for receptor binding and signaling activity. Therefore, this recombinant EPO is highly unlikely to achieve correct tertiary structure or possess any meaningful biological activity, though it may maintain some secondary structural elements.
1. Antibody Development and Validation Studies
This recombinant EPO serves as an excellent immunogen for generating antibodies against the polypeptide backbone of cynomolgus monkey EPO. The full-length mature protein sequence ensures comprehensive linear epitope coverage. However, antibodies produced against this non-glycosylated form may not efficiently recognize the native, glycosylated EPO found in biological systems, particularly in assays requiring conformational epitopes.
2. Comparative Species Analysis and Cross-Reactivity Testing
This protein can be used for limited comparative studies focusing on polypeptide sequence differences between species. However, for functional or immunological comparisons that depend on glycosylation patterns (which significantly influence half-life and immunogenicity), the data will be misleading as it lacks the natural post-translational modifications.
3. Biochemical Characterization and Stability Studies
This is an essential application for understanding the properties of this specific recombinant protein. Techniques like circular dichroism can assess secondary structure content, while thermal shift assays can determine stability under various conditions. However, the results will reflect the behavior of a non-glycosylated EPO variant, not the native glycosylated protein.
Final Recommendation & Action Plan
This recombinant EPO is valuable for immunological applications and biochemical characterization, but is fundamentally unsuitable for any functional studies due to the lack of essential glycosylation. The recommended approach is to prioritize Application 3 (Biochemical Characterization) to establish the protein's stability and structural properties, then proceed with Application 1 (Antibody Development) for generating sequence-specific antibodies. Application 2 should be limited to polypeptide-level comparisons only. Non-glycosylated EPO cannot form the proper tertiary structure required for specific interaction with the EPO receptor (EPOR). It may exhibit non-specific binding or fail to recognize genuine interaction partners, making any interaction data biologically irrelevant and misleading. For functional EPO studies, alternative approaches using mammalian expression systems (e.g., CHO cells) that provide proper glycosylation are necessary.