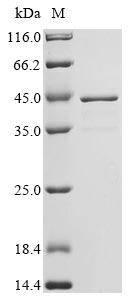
Recombinant Enterobacteria phage T4 Single-stranded DNA-binding protein (32) is expressed in E.coli and spans the full length of 301 amino acids. This protein comes engineered with an N-terminal 10xHis tag and a C-terminal Myc tag, which helps with purification and detection. It reaches a purity exceeding 90% as verified by SDS-PAGE, suggesting high-quality results for research applications.
The Single-stranded DNA-binding protein (32) from Enterobacteria phage T4 appears to play a critical role in DNA replication, recombination, and repair processes. It binds to single-stranded DNA, stabilizing it and preventing degradation—something that seems essential during phage replication. Molecular biology researchers find this protein particularly interesting because of its function in maintaining DNA integrity and supporting various DNA metabolic processes.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Enterobacteria phage T4 Single-stranded DNA-binding protein (gp32) is a viral protein that requires precise folding and proper nucleic acid-binding domain formation for its functional activity in DNA replication and repair. The E. coli expression system is homologous to this bacteriophage protein, significantly increasing the probability of correct folding. The dual N-terminal 10xHis-tag and C-terminal Myc-tag are relatively small and positioned at opposite ends, minimizing interference with the protein's functional domains. Given that gp32 has been successfully expressed in E. coli in numerous studies, this recombinant protein has a high probability of being correctly folded and functionally active.
1. Protein-Protein Interaction Studies Using Pull-Down Assays
This application is highly suitable for proper folding validation. gp32 interactions with replication proteins require precise tertiary structure. The homologous expression system supports proper folding for authentic protein-protein interactions. If correctly folded (verified), the protein is excellent for identifying physiological interaction partners in the T4 replisome. If misfolded/unverified (less likely), there is a risk of non-specific binding.
2. Antibody Development and Validation
Antibody development relies on antigenic sequence recognition. The full-length protein with high purity provides comprehensive epitope coverage for antibody production. If correctly folded (verified), the protein excels for generating conformation-sensitive antibodies. If misfolded/unverified, it remains suitable for producing antibodies against linear epitopes.
3. Biochemical Characterization and Stability Studies
Biochemical characterization is critical for validating protein quality and functional competence. If correctly folded (verified), characterization provides reliable data on DNA-binding properties, oligomerization, and stability. If misfolded/unverified, analysis still yields valuable physical property data.
4. Tag-Based Detection Assay Development
Tag-based detection relies on tag antigenicity rather than native protein folding. This application is highly reliable regardless of folding status. The dual tags provide flexible detection options for developing and optimizing immunoassays. The protein serves as an excellent standard for method validation.
Final Recommendation & Action Plan
The homologous E. coli expression system provides optimal conditions for this bacteriophage protein, resulting in a high probability of correct folding and functional activity. Begin with Application 3 (Biochemical Characterization) to validate folding quality through DNA-binding assays, size-exclusion chromatography, and thermal stability studies. Once correct folding and DNA-binding activity are confirmed, proceed confidently with Applications 1, 2, and 4 for interaction studies, antibody development, and assay development. The high purity and well-characterized tags make this protein particularly valuable for all proposed applications. For reliable results, always include appropriate DNA-binding controls in functional assays to confirm activity before proceeding to downstream applications.