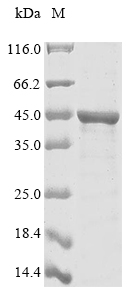
This recombinant Chlamydia trachomatis Major outer membrane porin, serovar B (ompA) comes from an E. coli expression system and contains the full length of the mature protein (23-394aa). The protein carries both an N-terminal 10xHis-tag and C-terminal Myc-tag, which makes purification and detection more straightforward. SDS-PAGE analysis shows purity levels exceeding 85%, suggesting it should work well for different research applications.
The major outer membrane porin (ompA) of Chlamydia trachomatis appears to be essential for maintaining the bacterial outer membrane's structural integrity and controlling what passes through it. This component likely helps keep the membrane stable while managing the transport of small molecules. Researchers studying bacterial pathogenesis and looking for new therapeutic targets may find understanding its function and how it interacts with host cells particularly valuable.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Chlamydia trachomatis Major outer membrane porin (OmpA) is a bacterial membrane protein that requires precise folding, beta-barrel formation, and proper membrane integration for its porin activity. The E. coli expression system is homologous to this bacterial membrane protein and contains the necessary machinery for outer membrane protein folding, increasing the probability of correct beta-barrel formation. However, membrane proteins present additional folding challenges, and the dual N-terminal 10xHis tag and C-terminal Myc tag may interfere with proper membrane integration or beta-barrel assembly. While E. coli expression provides favorable conditions for bacterial membrane proteins, experimental validation remains essential to confirm structural integrity and porin activity.
1. Antibody Development and Validation Studies
This application is highly suitable as antibody development primarily relies on antigenic sequence recognition. If correctly folded (as verified), the protein excels at generating conformation-sensitive antibodies that recognize native OmpA epitopes. If misfolded/unverified, it remains suitable for producing antibodies against linear epitopes, which are still valuable for diagnostic applications.
2. Protein-Protein Interaction Studies
Membrane protein interactions depend on correct beta-barrel formation and surface epitope presentation. This application requires proper folding validation. Porin interactions with host receptors or other bacterial proteins require precise tertiary structure. If correctly folded (verified), the protein is suitable for identifying physiological interaction partners. If misfolded/unverified, there is a high risk of non-specific binding or interaction failure.
3. Immunological Assay Development
Assay development relies on consistent antigen availability and detection, not functional conformation. This application is well-suited regardless of folding status. Immunoassays depend on antibody-epitope binding rather than functional activity. If correctly folded (verified), the protein provides authentic antigenic presentation. If misfolded/unverified, it remains effective as a standardized antigen for assay development.
4. Biochemical Characterization Studies
These studies are essential for determining folding status. Biochemical characterization provides valuable physical property (oligomerization, stability, and membrane integration properties) data for validating membrane protein folding and quality, not the native OmpA.
Final Recommendation & Action Plan
The E. coli expression system provides a suitable homologous environment for this bacterial membrane protein, but experimental validation of structural integrity is essential before functional applications. Begin with Application 4 (Biophysical Characterization) to assess folding quality through size-exclusion chromatography (oligomerization state), circular dichroism spectroscopy (beta-sheet content), and liposome reconstitution assays to test porin activity. Once correct folding and membrane integration are verified, proceed cautiously with Application 2 for interaction studies. Applications 1 and 3 (antibody development and immunoassays) can proceed immediately regardless of folding status. If misfolding is detected, limit applications to linear epitope antibody production and immunoassay standardization, avoiding all functional interaction studies. For reliable OmpA research, consider incorporating detergent screening and membrane mimetic systems to maintain protein stability during experiments.