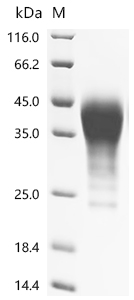
Recombinant Mouse Leukemia Inhibitory Factor (Lif) is produced using a mammalian cell expression system. The resulting protein includes the full-length sequence spanning amino acids 1-203, with a C-terminal 10xHis-tag that makes purification and detection more straightforward. SDS-PAGE analysis indicates purity levels exceeding 95%, which should provide reliable performance in research settings. This protein is designed strictly for research use.
Leukemia Inhibitory Factor (Lif) functions as a cytokine that appears to influence several cellular processes - cell differentiation, proliferation, and survival among them. The protein likely plays an important role in regulating immune responses and hematopoiesis. Perhaps most notably, Lif seems crucial for keeping embryonic stem cells in their undifferentiated state, which makes it particularly valuable for researchers working in developmental biology and regenerative medicine.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Mammalian cells provide a suitable environment for proper folding, disulfide bond formation, and potential glycosylation of eukaryotic proteins like LIF, which requires dimerization and correct conformation for bioactivity (e.g., binding to LIF receptor and gp130). The C-terminal His tag is less likely to interfere with functional domains. The high purity indicates low impurities. However, without experimental validation (e.g., cell-based bioactivity assays), the protein cannot be assumed to be bioactive, though the expression system suggests a high probability of correct folding.
1. Antibody Development and Validation Studies
This recombinant LIF is suitable as an immunogen for generating antibodies, as mammalian cell expression likely preserves native epitopes. The His tag facilitates purification and screening. However, validate antibody specificity against native LIF from mouse cells or tissues to ensure recognition of physiological conformations, even if folding is probable.
2. Protein-Protein Interaction Studies
The His tag enables pull-down assays to study interactions (e.g., with LIF receptor). If LIF is bioactive (verified via activity assays), interactions are likely physiological. If unverified, misfolding could lead to non-specific binding. Validate interactions with native LIF or functional assays.
3. His-Tag Mediated Purification Method Development
This application is feasible regardless of folding status, as it focuses on tag-based purification optimization. The protein can be used to test nickel-affinity resins, buffers, or elution conditions without requiring bioactivity.
4. Comparative Species-Specific Binding Studies
If LIF is confirmed bioactive (e.g., via receptor binding assays), it can be used for comparative binding studies across species. Without validation, binding data may be unreliable due to potential misfolding. Always include positive controls with known active LIF.
Final Recommendation & Action Plan
Before using this recombinant LIF for functional applications, validate its bioactivity through a cell-based assay (e.g., inhibition of embryonic stem cell differentiation or STAT3 phosphorylation). If active, proceed with interaction or binding studies; if inactive, limit use to non-functional applications like antibody production (with validation against native LIF). For purification method development, use directly without validation. Always include appropriate controls to ensure biological relevance.