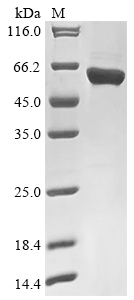
Recombinant Rabies virus Nucleoprotein (N) is produced in an E. coli expression system, spanning the complete sequence from amino acids 1 to 450. The protein carries an N-terminal 10xHis tag and a C-terminal Myc tag, which aid in purification and detection processes. SDS-PAGE analysis indicates purity levels above 90%, and this reagent is intended strictly for research use, maintaining low endotoxin levels to support experimental accuracy.
The rabies virus nucleoprotein (N) appears to serve a crucial function in the viral life cycle. It's primarily responsible for wrapping around the viral RNA genome and forming the ribonucleoprotein complex. This protein seems essential for virus replication and transcription, which is why it has become a key focus in virology research. Studying this protein may offer valuable insights into viral assembly mechanisms and could potentially lead to new therapeutic approaches.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Rabies virus nucleoprotein (N) is a structural protein that forms a complex with viral RNA and requires proper oligomerization (likely forming trimers or higher-order structures) for its function in ribonucleoprotein complex formation. The E. coli expression system can produce soluble proteins, but the dual N-terminal and C-terminal tags may sterically interfere with proper oligomerization and folding. While the full-length protein (1-450aa) is present, the tags might prevent native quaternary structure formation. The probability of correct folding and functional activity (e.g., RNA binding) is moderate but requires experimental validation.
1. Antibody Development and Validation Studies
This recombinant N protein serves as an excellent immunogen for generating antibodies against the rabies virus nucleoprotein. The full-length sequence ensures comprehensive coverage of the epitope. The dual tags facilitate purification and screening. However, antibodies may not efficiently recognize conformational epitopes dependent on proper oligomerization in the native viral context.
2. Protein-Protein Interaction Studies
Protein-protein interactions depend on precise quaternary structure. If correctly folded and oligomerized, the protein could identify physiological partners (e.g., viral phosphoprotein P). However, misfolding or tag interference may lead to non-specific binding or failure to interact. The C-terminal Myc tag might sterically block interaction sites. Data require validation with an endogenous protein.
3. ELISA-Based Detection System Development
This protein is highly suitable as a standard for quantitative ELISA to detect anti-nucleoprotein antibodies. The assay depends on antibody binding to linear epitopes, so the protein's oligomerization state is less critical. The tags enable consistent immobilization for reliable standard curves.
4. Biochemical Characterization and Stability Studies
This is the essential first step to assess protein quality. Techniques like size-exclusion chromatography with multi-angle light scattering (SEC-MALS) can determine oligomeric state and homogeneity. Circular dichroism can be used to analyze secondary structure and thermal stability. These studies provide critical data on whether the protein forms native-like oligomers.
Final Recommendation & Action Plan
The dual-tagged recombinant N protein has potential for immunological and biochemical applications but requires validation of oligomerization before reliable use in interaction studies. The immediate priority is Application 4 (Biochemical Characterization) to assess oligomeric state via SEC-MALS and folding quality. If the protein shows native-like oligomerization (e.g., trimer formation), proceed to validate RNA-binding activity. Once oligomerization is confirmed, Application 2 (Interaction Studies) can be considered with caution. Applications 1 and 3 (Antibody Development and ELISA) can proceed immediately. For functional studies, consider tag removal or use an endogenous protein from viral particles. This systematic approach ensures a reliable interpretation of results.