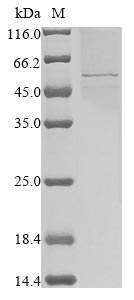
Recombinant Human coronavirus 229E Nucleoprotein (N) is produced using a baculovirus expression system, which appears to ensure high-quality protein production. The full-length protein spans 1-389 amino acids and comes with an N-terminal 10xHis-tag and a C-terminal Myc-tag for easier purification and detection. SDS-PAGE analysis shows the purity exceeds 85%, making this recombinant protein suitable for various research applications that demand high-quality reagents.
Human coronavirus 229E's Nucleoprotein (N) plays a critical role in the viral replication cycle. It participates in packaging viral RNA and forming the ribonucleoprotein complex—both essential for efficient viral transcription and replication. Studying this protein may be crucial for understanding coronavirus biology mechanisms and could potentially lead to therapeutic strategies.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The protein is expressed in a baculovirus system (eukaryotic, supporting native-like folding and post-translational modifications [PTMs]—critical for the full-length HCoV-229E nucleoprotein [N] to interact with viral RNA and host factors). Full-length expression (1–389 aa) preserves all functional domains, and dual tags (10xHis-N-terminal, Myc-C-terminal) minimally disrupt structure. However, no direct validation of folding (e.g., circular dichroism for secondary structure, thermal shift assays for stability) or native bioactivity (e.g., RNA binding affinity, oligomerization, or host protein interactions) is provided. While baculovirus expression strongly suggests correct folding, bioactivity remains unconfirmed, limiting definitive claims about its native functionality.
1. Antibody Development and Validation Studies
This full-length recombinant HCoV-229E N protein can serve as an immunogen for generating monoclonal/polyclonal antibodies, and the dual tags simplify purification/detection. However, antibody specificity must be validated against native N—baculovirus expression preserves conformational epitopes, but tag presentation may alter antigenicity, leading to cross-reactivity with non-native targets. ELISA can screen for affinity, but validation with native protein or infected cells is critical.
2. Protein-Protein Interaction Studies
Pull-down assays using the His/Myc tags can identify host interactors, but results depend on correct folding—baculovirus expression improves native folding, but interactions must be validated via co-IP or functional assays to rule out artifacts from misfolding. The full length preserves all potential domains, but bioactivity (e.g., RNA binding) is unconfirmed.
3. Structural and Biochemical Characterization
This protein supports biochemical assays (e.g., thermal stability, oligomerization) and structural studies (e.g., circular dichroism, analytical ultracentrifugation). Baculovirus expression ensures native-like structure, but the C-terminal Myc tag may interfere with high-resolution techniques (e.g., crystallography)—tag cleavage (via protease) may improve results. RNA binding and nucleocapsid assembly must be validated experimentally.
4. Comparative Coronavirus Research
This baculovirus-expressed N protein enables cross-reactivity studies with other coronavirus N proteins, as standardized folding reduces variability. However, PTMs may differ slightly between species—results should be contextualized, and direct comparisons (e.g., antibody cross-reactivity) must account for evolutionary divergence.
5. Assay Development and Optimization
The dual-tagged N protein is a useful immunoassay standard, but its native conformation must be confirmed—the tags enable consistent detection, but assay performance (sensitivity/specificity) may differ if the protein misfolds. Validate with native N or clinical samples to ensure relevance to HCoV-229E biology.
Final Recommendation & Action Plan
This baculovirus-expressed, full-length HCoV-229E N protein is a strong candidate for antibody development, immunoassays, and comparative research due to its eukaryotic folding. Prioritize validation of folding (CD/thermal shift) and bioactivity (RNA binding/oligomerization) to ensure reliability. For interactions or structural studies, use tag cleavage if needed and pair with co-IP/functional assays. If validation passes, leverage its consistency for downstream applications—always include native N controls to contextualize results. If folding/bioactivity fails, optimize expression (e.g., co-express chaperones) or use a viral replication system to produce native protein.