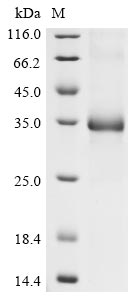
Recombinant Human coronavirus HKU1 Spike glycoprotein (S) is produced in E. coli and consists of a partial length from amino acids 310 to 622. The protein includes a C-terminal 6xHis-tag for ease of purification and detection. Purity reaches greater than 90% as determined by SDS-PAGE, which appears to make it suitable for various research applications. This product is intended for research use only, with no endotoxin level specified.
The Spike glycoprotein (S) of Human coronavirus HKU1 seems integral to the virus's ability to attach and enter host cells. It likely plays a crucial role in viral entry through interactions with host cell receptors, making it a key focus in studies of viral infection mechanisms and immune response. Understanding its structure and function may be essential for developing therapeutic strategies and vaccines against coronaviruses.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
While the His tag improves solubility, E. coli struggles to correctly fold eukaryotic membrane proteins like the S protein. No data confirms native secondary/tertiary structure (e.g., circular dichroism for disulfide bonds, thermal shift assays for stability). E. coli may misfold the fragment, leading to aggregation or non-functional conformations. The fragment lacks full-length S context (e.g., receptor-binding domain [RBD], fusion machinery), so it cannot recapitulate native interactions (e.g., with host receptors like CEACAM5 for HKU1). Bioactivity (e.g., ligand binding, oligomerization) is untested and unlikely to mirror wild-type S.
1. Antigen for Antibody Development and Characterization
This recombinant S fragment (310–622 aa) can serve as an antigen for generating antibodies, but antibody specificity must be validated against native S—E. coli-expressed protein may present non-native epitopes, leading to cross-reactivity with irrelevant targets. The His tag streamlines purification/immobilization for immunization, but antibodies may not recognize the fragment in its native context (e.g., on viral particles). High purity supports consistent immunogenicity, but validation with native protein is critical.
2. Protein-Protein Interaction Studies
Pull-down assays using the His tag can identify interactors, but results depend on correct folding—E. coli-expressed fragments often misfold, causing false positives/negatives. Identified partners (e.g., host receptors or viral NSPs) must be validated via co-IP or functional assays (e.g., replicon assays) to rule out artifacts. The partial length restricts insights to interactions within this domain, not full-length S biology.
3. Structural and Biochemical Characterization
This fragment supports preliminary biophysical studies (e.g., circular dichroism for secondary structure, dynamic light scattering for stability) but cannot inform native S architecture—E. coli-expressed protein may lack correct disulfide bonds or domain folding. Structural conclusions (e.g., oligomerization) must be contextualized by folding limitations.
4. ELISA-Based Binding and Competition Assays
Immobilization of the His-tagged protein enables quantitative binding studies, but misfolding may alter binding kinetics—results are qualitative at best. Quantitative affinity measurements require a confirmed native structure, as the fragment’s conformation may not support native-like interactions. High purity ensures consistent coating, but validation with native S is needed for assay relevance.
Final Recommendation & Action Plan
This E. coli-expressed HCoV-HKU1 S fragment has potential for antibody development or preliminary interaction studies, but requires rigorous validation first, confirm folding via circular dichroism/thermal shift assays to rule out misfolding; second, test bioactivity (e.g., binding to known ligands or host receptors) using co-IP or cell-based assays. Optimize expression (e.g., co-express chaperones like GroEL/ES, lower induction temperature) to improve solubility/native folding. For antibody development, validate specificity against native S; for interactions/ELISA, use tag cleavage or orthogonal methods (e.g., surface plasmon resonance) to reduce artifacts. If folding/bioactivity fails, switch to a eukaryotic system (e.g., mammalian/insect cells) to ensure native structure—this fragment’s utility for functional studies depends on validating its structural and functional integrity relative to the full-length S protein.