Purity
Greater than 90% as determined by SDS-PAGE.
Endotoxin
Less than 1.0 EU/ug as determined by LAL method.
Activity
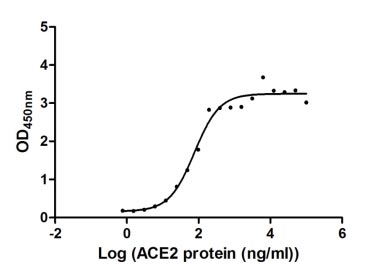
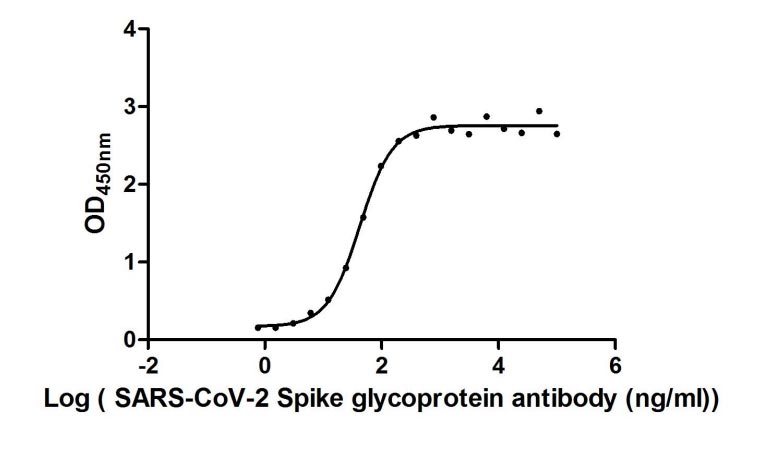
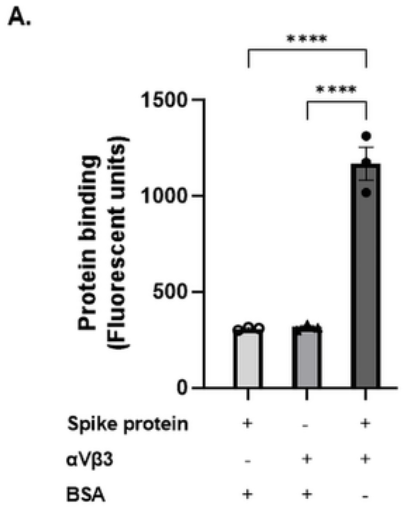
①Measured by its binding ability in a functional ELISA. Immobilized SARS-CoV-2-S at 2 μg/ml can bind human ACE2 (CSB-MP866317HU), the EC50 of SARS-CoV-2-S protein is 56.64 - 103.6 ng/ml.②Measured by its binding ability in a functional ELISA. Immobilized SARS-CoV-2-S at 2 μg/ml can bind SARS-CoV-2-S Antibody (CSB-RA33245A1GMY), the EC50 of SARS-CoV-2-S protein is 36.79-48.87 ng/ml
Research Area
Microbiology
Alternative Names
S; 2; Spike glycoprotein; S glycoprotein; E2; Peplomer protein)
Species
Severe acute respiratory syndrome coronavirus 2 (2019-nCoV) (SARS-CoV-2)
Expression Region
16-685aa
Target Protein
Sequence
VNLTTRTQLPPAYTNSFTRGVYYPDKVFRSSVLHSTQDLFLPFFSNVTWFHAIHVSGTNGTKRFDNPVLPFNDGVYFASTEKSNIIRGWIFGTTLDSKTQSLLIVNNATNVVIKVCEFQFCNDPFLGVYYHKNNKSWMESEFRVYSSANNCTFEYVSQPFLMDLEGKQGNFKNLREFVFKNIDGYFKIYSKHTPINLVRDLPQGFSALEPLVDLPIGINITRFQTLLALHRSYLTPGDSSSGWTAGAAAYYVGYLQPRTFLLKYNENGTITDAVDCALDPLSETKCTLKSFTVEKGIYQTSNFRVQPTESIVRFPNITNLCPFGEVFNATRFASVYAWNRKRISNCVADYSVLYNSASFSTFKCYGVSPTKLNDLCFTNVYADSFVIRGDEVRQIAPGQTGKIADYNYKLPDDFTGCVIAWNSNNLDSKVGGNYNYLYRLFRKSNLKPFERDISTEIYQAGSTPCNGVEGFNCYFPLQSYGFQPTNGVGYQPYRVVVLSFELLHAPATVCGPKKSTNLVKNKCVNFNFNGLTGTGVLTESNKKFLPFQQFGRDIADTTDAVRDPQTLEILDITPCSFGGVSVITPGTNTSNQVAVLYQDVNCTEVPVAIHADQLTPTWRVYSTGSNVFQTRAGCLIGAEHVNNSYECDIPIGAGICASYQTQTNSPRRAR
Tag Info
N-terminal 10xHis-tagged and C-terminal Flag-tagged
Form
Lyophilized powder
Note: We will preferentially ship the format that
we have in stock, however, if you have any special requirement for the format, please remark your
requirement when placing the order, we will prepare according to your demand.
Buffer
Lyophilized from a 0.2 μm sterile filtered 20 mM Tris-HCl, 0.5 M NaCl, 6% Trehalose, pH 8.0
Reconstitution
We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20°C/-80°C. Our default final concentration of glycerol is 50%. Customers could use it as reference.
Storage Condition
Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid
repeated freeze-thaw
cycles.
Shelf Life
The shelf life is related to many factors, storage state, buffer ingredients, storage
temperature
and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized
form is 12 months at -20°C/-80°C.
Lead Time
3-7 business days
Notes
Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
Datasheet & COA
Please contact us to get it.